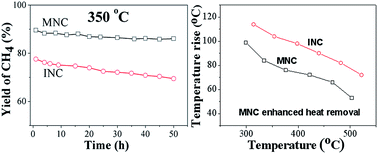
The synthesis and characterization of highly active and stable Ni nanoparticles supported on macro–mesoporous Al2O3 as a CO methanation catalyst for the production of synthetic natural gas are reported. The macro–mesoporous Ni–Al2O3 catalyst (MNC) is synthesized by combination of sol–gel and supercritical drying methods. The MNC showed much higher activity and higher thermal stability for methanation than the catalyst obtained via the conventional wet impregnation method, especially under harsh conditions of high temperatures and high GHSV (90 000 h−1). The enhancement in activity of the synthesized MNC catalyst is attributed to the highly stable and smaller Ni nanoparticles that are embedded on the macro–mesoporous structure. The MNC also showed a higher rate constant and lower diffusion activation energy, which should be attributed to the macro–mesoporous structure that facilitated the diffusion of gas products and then enhanced the removal of reaction heat from the catalyst surface. Thus leading to a higher resistance to Ni sintering and carbon deposition.