A facile synthesis of isoxazolo[3,4-a]pyrrolizine and isoxazolo[4,3-c]pyridine derivatives via intramolecular nitrone cycloaddition reaction†
Manickam Bakthadoss*ab and
Jayakumar Srinivasanb
aDepartment of Chemistry, Pondicherry University, Pondicherry-605014, India. E-mail: bhakthadoss@yahoo.com; Fax: +91-413-2654830
bDepartment of Organic Chemistry, University of Madras, Guindy Campus, Chennai-600025, Tamil Nadu, India
First published on 24th July 2015
Abstract
A facile and efficient protocol for the construction of isoxazolo[3,4-a]pyrrolizine and isoxazolo[4,3-c]pyridine derivatives through in situ formation of nitrone ylide followed by an intramolecular [3 + 2] cycloaddition reaction is described. This reaction paved the pathway way for the successful assembly of angularly substituted cyclic heterocycles, which creates two new rings, three contiguous stereocenters and one tetra substituted carbon center in a highly diastereoselective fashion with good yields.
The development of highly efficient strategies for the synthesis of isoxazolidines is very important since isoxazolidines represent an important class of versatile intermediates for a wide range of natural products and pharmaceuticals. Notably, these five-membered ring heterocycles can readily be converted into numerous useful functional groups such as amino acids, β-lactams, and 1,3-amino alcohols through reductive N–O bond cleavage. Therefore, it is not surprising that great efforts have been devoted to the development of new methods for the construction of isoxazolidine core. Among the different approaches available for the preparation of this structural motifs, [3 + 2] cycloaddition of nitrones with alkenes is one of the most straightforward and convenient method1 which leads to interesting structural units.
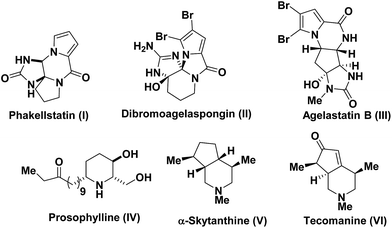
Naturally-occurring pyrrole alkaloids and their derivatives have attracted significant attentions due to their bioactivities such as antibacterial activity and antimicrobial activities.2 Important representatives of the naturally-occurring pyrrole fused tricylic alkaloids such as phakellstatin (I), dibromoagelaspongin (II) and agelastatin B (III) are shown in Fig. 1.3 Similarly, piperidine derivatives are very important in synthetic and medicinal chemistry due to their occurrence in numerous biologically relevant alkaloids and pharmaceutical agents.4 Typical examples include antibiotic and anesthetic prosopis alkaloid prosophylline (IV), α-skytanthine (V) and tecomanine (VI) which displays a variety of biological activities including neurogenic inflammation, pain transmission, and regulation of the immune response (Fig. 1).5
To the best of our knowledge,6 the Baylis–Hillman derivatives have not been utilized for the synthesis of fused tricyclic tetrahydro-1H-isoxazolo[3,4-a]pyrrolizine and octahydro isoxazolo[4,3-c]pyridine via 1,3-dipolar nitrone cycloaddition to date. Therefore, in our continuous effort towards the synthesis of highly functionalized poly heterocyclic compounds,7 we speculated that the Baylis–Hillman derivatives can be effectively employed as a key starting material for the synthesis of complex angularly substituted tetrahydro-1H-isoxazolo[3,4-a]pyrrolizine and octahydroisoxazolo[4,3-c]pyridine frameworks via intramolecular 1,3-dipolar nitrone cycloaddition reaction.
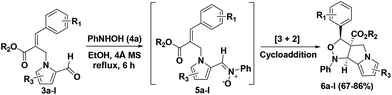
We envisaged that treatment of Baylis–Hillman acetate (1) with pyrrole-2-carboxaldehyde (2) will leads to (E)-methyl 2-((2-formyl-1H-pyrrol-1-yl)methyl)-3-phenylacrylate (3) and further treatment of N-allylated aldehyde (3) with N-phenylhydroxylamine (4) will afford the tricyclic tetrahydro-1H-isoxazolo[3,4-a]pyrrolizine (6) with ester functionality in the ring junction as depicted in the retrosynthetic strategy (Scheme 1).
To execute our idea we treated N-allylated aldehyde 3a (prepared from BH acetate with 2) and PhNHOH (4a) with 4 Å MS in ethanol as solvent under reflux condition over a period of 6 h which successfully led to the desired isoxazolo[3,4-a]pyrrolizine (6a) containing ester functionality at the angular position in very good yield (80%). The reaction proceeds through an in situ formation of nitrone (5a) followed by [3 + 2] cycloaddition reaction sequence as shown in Table 1.
| a All reactions were carried out on 1 mmol scale of N-allylated derivatives (3a–l) with 1.1 mmol of PhNHOH (4a) with 4 Å MS in EtOH (10 mL) as a solvent at reflux temperature for 6 h.b Isolated yield of the pure product.c Structure of the molecule was further confirmed by single-crystal X-ray data.8 |
|---|
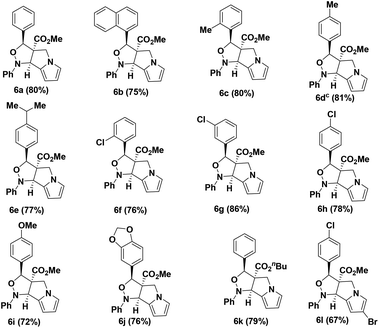 |
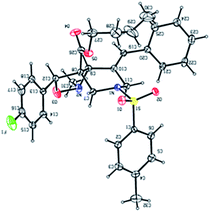
Encouraged by this result, we have utilized variety of N-allylated aldehydes (3b–l), with PhNHOH (4a) under similar reaction condition which smoothly led to the corresponding tricyclic tetrahydro-1H-isoxazolo[3,4-a]pyrrolizines (6b–l) possessing ester functionality at the angular position in good yields (67–86%) and the results are summarized in Table 1. Brominated pyrrole derivative (3l) also utilized for cycloaddition reaction and provided the corresponding cycloadduct 6l (67% yield) which will be useful for further elaboration in pyrrole unit. The structure of the molecule 6d was confirmed using single crystal X-ray analysis. The crystal structure of compound 6d shows that the phenyl group and the adjacent ester moiety adopt an anti-orientation, which is presumably due to the initial trans geometry of the phenyl group and ester moiety present in the double bond at the vicinal positions of compound 3d (Fig. 2). The ring junction ester and hydrogen are in cis orientation.
After the successful synthesis of tetrahydro-1H-isoxazolo[3,4-a]pyrrolizine frameworks, we focussed our attention towards the synthesis of octahydroisoxazolo[4,3-c]pyridine derivatives from the MBH adducts of acrylates which will undergo intramolecular nitrone cycloaddition to furnish isoxazolo[4,3-c]pyridine (13) as shown in the retrosynthetic strategy (Scheme 2).
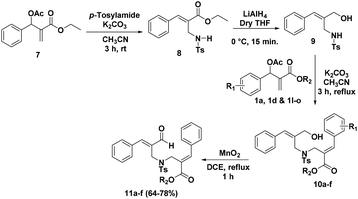
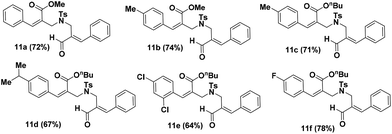
The approach for the synthesis of isoxazolo[4,3-c]pyridine (13) involves the utilization of BH acetate 7, which was transformed to allylamine 8 in a stereo selective fashion via SN′2 reaction with tosylamine in the presence of K2CO3 in CH3CN for 3 h at room temperature. In order to reduce the ester functionality into alcohol group, allylamine 8 was treated with LiAlH4 in THF at 0 °C for 15 minutes which afforded the corresponding alcohol 9. The reaction of substituted alcohol 9 with BH acetates (1) in the presence of K2CO3 in CH3CN at reflux temperature for 3 h afforded allylic alcohol 10a in a chemo selective fashion. Further oxidation of allylic alcohol 10a in presence of MnO2 afforded the aldehyde compound 11a in 72% yield (Table 2). After successfully obtained the requisite aldehyde 11a, we prepared variety of aldehyde derivatives 11b–f in good yields (64–78%) with a view to carry out the nitrone cycloaddition reaction.
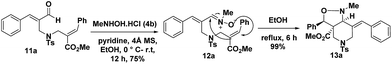
In order to synthesize the cycloadduct 13a, the requisite precursor 11a was treated with N-methylhydroxylamine hydrochloride (4b) in the presence of pyridine as a base with 4 Å MS in ethanol under reflux condition for 6 h afforded a desired product 13a in 83% yield. Encouraged by the result, we carried out the cycloaddition reaction of substrates 11b–f with N-methyl hydroxylamine hydrochloride (4b) successfully afforded a desired isoxazolo[4,3-c]pyridines (13b–f) in 80–86% yields (Table 3). The structure of the compound 13f was further confirmed using single crystal X-ray analysis (Fig. 3).
| a All reactions were carried out on 1 mmol scale of aldehydes (11a–f) with 1.1 mmol of MeNHOH·HCl (4b) presence of pyridine with 4 Å MS in allowed to stir in EtOH (10 mL) at reflux temperature for 6 h.b Isolated yields of the pure product.c The structure of the molecule was further confirmed by single-crystal X-ray data.8 |
|---|
 |
We believe that the reaction proceeds via the formation of nitrone followed by intramolecular [3 + 2] cycloaddition reaction. To confirm the intermediate, we have isolated the nitrone intermediate (75%) by following the reaction condition depicted in Scheme 3. The nitrone intermediate 12a was refluxed in EtOH for 6 h which successfully led to the desired cycloadduct 13a in 99% yield.
Conclusions
We have successfully developed a simple and novel protocol for the facile synthesis of complex angularly substituted frameworks containing a isoxazolo[3,4-a]pyrrolizine and isoxazolo[4,3-c]pyridine ring system involving in situ formation of nitrone followed by an intramolecular 1,3-dipolar nitrone cycloaddition reaction using Baylis–Hillman derivatives for the first time. The new [3 + 2] cycloaddition reaction leads to a novel class of angularly substituted fused bicyclic/tricyclic isoxazolo[4,3-c]pyridine and isoxazolo[3,4-a]pyrrolizine, creating two rings, three contiguous stereocenters, one of them being a tetra substituted carbon center in a unique fashion.Acknowledgements
We thank DST-SERB for the financial support. We also thank DST-FIST for the NMR facility. J. S. thanks CSIR for his SRF.Notes and references
- R. C. F. Jones and J. N. Martin, in Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products, ed. A. Padwa and W. H. Pearson, John Wiley & Sons, New York, 2002, pp. 1–81 Search PubMed.
- (a) F. Cafieri, E. Fattorusso and O. Tagliatatela-Scafati, J. Nat. Prod., 1998, 61, 122 CrossRef CAS PubMed; (b) F. Cafieri, R. Carnuccio, E. Fattorusso, O. Tagliatatela-Scafati and T. Vallefuoco, Bioorg. Med. Chem. Lett., 1997, 7, 2283 CrossRef CAS.
- (a) G. P. Pettit, J. McNulty, L. Herald, D. L. Doubek, J.-C. Chapuis, J. M. Schmidt, L. P. Tackett and M. P. Boyd, J. Nat. Prod., 1997, 60, 180 CrossRef CAS PubMed; (b) S. Wang and D. Romo, Angew. Chem., Int. Ed., 2008, 47, 1284 CrossRef CAS PubMed; (c) Z. Jin, Nat. Prod. Rep., 2011, 28, 1143 RSC.
- (a) L.-X. Liu, Y.-P. Ruan, Z.-Q. Guo and P.-Q. Huang, J. Org. Chem., 2004, 69, 6001 CrossRef CAS PubMed; (b) S. Vidyacharan, A. H. Shinde, B. Satpathi and D. S. Sharada, Green Chem., 2014, 16, 1168 RSC; (c) J. M. Andrés, R. Pedrosa and A. Pérez-Encabo, Tetrahedron Lett., 2006, 47, 5317 CrossRef PubMed; (d) A. S. González, R. G. Arrayás, M. R. Rivero and J. C. Carretero, Org. Lett., 2008, 10, 4335 CrossRef PubMed; (e) S. Prévost, P. Phansavath and M. Haddad, Tetrahedron: Asymmetry, 2010, 21, 16 CrossRef PubMed; (f) S.-T. Ruan, J.-M. Luo, Y. Du and P.-Q. Huang, Org. Lett., 2011, 13, 4938 CrossRef CAS PubMed; (g) C. K. Tan, C. le and Y.-Y. Yeung, Chem. Commun., 2012, 48, 5793 RSC; (h) S. V. Pansare and E. K. Paul, Org. Biomol. Chem., 2012, 10, 2119 RSC; (i) S. Vidyacharan, A. Sagar, C. Chaitra and D. S. Sharada, RSC Adv., 2014, 4, 34232 RSC.
- (a) X. Q. Ma and D. R. Gang, Nat. Prod. Rep., 2004, 21, 752 RSC; (b) K. Kaneda and T. Honda, Tetrahedron, 2008, 64, 11589 CrossRef CAS PubMed; (c) M. C. Desai, S. L. Lefkowitz, P. F. Thadeio, K. P. Longo and R. M. Snider, J. Med. Chem., 1992, 35, 4911 CrossRef CAS.
- (a) E. M. Beccalli, G. Broggini, G. La Rosa, D. Passarella and T. Pilati, J. Org. Chem., 2000, 65, 8924 CrossRef CAS PubMed; (b) A. Terraneo, G. Zecchi, A. Arnone, G. Broggini, D. Passarella, A. Terraneo and G. Zecchi, J. Org. Chem., 1998, 63, 9279–9284 CrossRef; (c) A. Mishra, N. Rastogi and S. Batra, Tetrahedron, 2012, 68, 2146 CrossRef CAS PubMed.
- (a) M. Bakthadoss, D. Kannan, J. Srinivasan and V. Vinayagam, Org. Biomol. Chem., 2015, 13, 2870 RSC; (b) M. Bakthadoss and D. Kannan, RSC Adv., 2014, 4, 11723 RSC; (c) M. Bakthadoss and G. Sivakumar, Tetrahedron Lett., 2014, 55, 1765 CrossRef CAS PubMed; (d) D. Basavaiah, M. Bakthadoss and G. J. Reddy, Synth. Commun., 2002, 32, 689 CrossRef CAS PubMed; (e) M. Bakthadoss, A. Devaraj and D. Kannan, Eur. J. Org. Chem., 2014, 1505 CrossRef CAS PubMed; (f) M. Bakthadoss, D. Kannan and R. Selvakumar, Chem. Commun., 2013, 49, 10947 RSC; (g) M. Bakthadoss and G. Murugan, Synth. Commun., 2008, 38, 3406 CrossRef CAS PubMed; (h) M. Bakthadoss, J. Srinivasan and R. Selvakumar, Aust. J. Chem., 2014, 67, 295 CAS; (i) M. Bakthadoss, G. Sivakumar and D. S. Sharada, Synthesis, 2013, 237–245 CAS; (j) M. Bakthadoss and G. Murugan, Eur. J. Org. Chem., 2010, 5825 CrossRef CAS PubMed; (k) M. Bakthadoss and A. Devaraj, Tetrahedron Lett., 2015, 56, 3954 CrossRef CAS PubMed.
- ESI†.
Footnote |
| † Electronic supplementary information (ESI) available: All experimental procedure, spectral data and copies of 1H and 13C NMR spectra and of all new compounds, X-ray structural data for compounds 6d & 13f. CCDC 1053095 and 1035588. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra10371a |
| This journal is © The Royal Society of Chemistry 2015 |