Facile iron(III)-mediated ATRP of MMA with phosphorus-containing ligands in the absence of any additional initiators†
Liangjiu Bai*a,
Wenxiang Wanga,
Hou Chena,
Lifen Zhangb,
Zhenping Cheng*b and
Xiulin Zhub
aSchool of Chemistry and Materials Science, Ludong University, Yantai 264025, China. E-mail: bailiangjiu@163.com
bJiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Department of Polymer Science and Engineering, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, China. E-mail: chengzhenping@suda.edu.cn
First published on 16th July 2015
Abstract
A series of phosphorus-containing ligands was employed to establish a novel polymerization system for the iron(III)-mediated polymerization of methyl methacrylate (MMA) just using FeCl3·6H2O or FeBr3 as the catalyst without any additional initiators and reducing agents. The polymerization results showed that this polymerization system involving MMA/FeX3 (X = Cl, Br)/phosphorus-containing ligand was a typical “living”/controlled radical polymerization process: first-order polymerization kinetics with respect to monomer concentration and a linear increase of the molecular weight of the resultant PMMAs with conversion while keeping a narrow molecular weight distribution. Chain end analysis of the obtained PMMA based on 1H NMR, 31P NMR were used to confirm the precise structure of the obtained polymers. The results showed that phosphorus-containing complexes acted as both ligand and thermal radical initiators in this process, which was consistent with a reverse atom transfer radical polymerization (reverse ATRP) mechanism.
Introduction
The development of atom transfer radical polymerization (ATRP) provides a ubiquitous tool for the synthesis of well-defined (co)polymers with precisely controlled functionalities, topologies and compositions.1 As a multicomponent system, ATRP is usually composed of monomers, an initiator, and a catalyst including a transition metal species with suitable ligands. Sometimes an additive such as solvent or reducing agent is also used. The catalyst plays a key role in an ATRP process. Many researchers have made great efforts to investigate the effect of various transition metal complexes on polymerization, such as copper,2 iron,3 ruthenium,4 and other transition metals.5 Among these ATRP metal catalysts, iron has attracted extensive attentions owing to their readily abundance, low toxicity and biocompatibility although iron complexes were generally considered to be inferior to copper or ruthenium complexes for the control of polymerization.6A recent advance in ATRP is the development of air-stable catalysts in their higher oxidation states (e.g., Cu(II), Fe(III) salts) and reducing the amount of catalyst to prepare well-defined polymers.7 The use of oxidatively stable catalysts overcomes the air-sensitive problem of lower oxidation state metals and makes the preparation and storage of ATRP catalyst systems more facile.8 Until now, several new ATRP techniques based on higher oxidation state catalysts including reverse ATRP (RATRP),9 simultaneous reverse and normal initiation (SR&NI ATRP),10 initiators for continuous activator regeneration (ICAR) ATRP,11 activators generated by electron transfer (AGET) ATRP,12 and activators regenerated by electron transfer (ARGET) ATRP,13 have been developed. In a common AGET ATRP process, the component includes at least monomer, ATRP initiator, higher oxidation state catalyst, ligand and reducing agent; therefore, it is a complicated multi-component system. The simple ATRP system based on higher oxidation state catalyst could be reverse ATRP where it is necessary in the presence of a thermal radical initiator besides catalyst and ligand. Is it possible to explore simpler ATRP system mediated by higher oxidation state catalyst?
Schubert et al. reported that the ATRP using copper(II) complex combination with ATRP initiator (ethyl 2-bromoisobutyrate, EBiB) resulted in well-defined PMMA.14 Recently, Noh and coworkers developed the iron(III)-catalyzed ATRP with phosphorus ligands in the absence of any conventional radical initiator or reducing agent but with the normal ATRP initiators.15 The components often include vinyl monomer, ATRP initiator, higher oxidation state iron(III)-catalyst and phosphorus-containing ligand. Various types of phosphorus-containing ligands can be used, including triphenylphosphine (TPP), tributylphosphine (TBP), 2-(diphenylphosphino) pyridine (DPPP), 2-(diphenylphosphino) benzaldehyde (DPPB), diphenyl-(2-methoxyphenyl) phosphine (DPMPP), 2-(diphenylphosphino)-N,N′-dimethyl-(1,1′-biphenyl)-2-amine (DPPDMA). As mentioned above, although the thermal radical initiator is not necessary for a successful metal-mediated living radical polymerization with higher oxidation state catalyst, the ATRP initiator such as EBiB is needed in these polymerization systems.
Mathias et al. successfully reported an air-induced reverse ATRP system catalyzed by Ni(II) or Cu(II) species without any additional thermal radical and ATRP initiators,16 and subsequently Matyjaszewski's group gave a deeper insight into the plausible mechanism of air (oxygen) initiation via synthesizing high molecular weight polymers in the presence of PIB-functionalized ATRP macroinitiator.17 Zhu and coworkers mentioned that the Cu(II)-mediated reverse ATRP of styrene without thermal radical initiator could be successfully carried out via spontaneous generation of radicals from styrene by a Mayo-type process at higher temperature (above 110 °C).18
On the other hand, as we all know, organic ligand plays a key role in a successful ATRP process in which it serves as a carrier of transition metal complex to facilitate the catalyst to dissolve in reaction media. Phosphorus-containing ligands are usually used as efficient ligands for the iron-mediated ATRP. However, we pay little attention to the fact that some phosphorus ligands such as TPP can act as a radical initiator for the polymerization of methyl methacrylate (MMA) as reported by Eldred and coworkers.19 Considering the advantages of iron catalyst and multifunction of phosphorus ligands, in this paper, we report a facile iron(III)-catalyzed polymerization system comprising only monomer (MMA), phosphorus-containing ligands (the structures shown in Scheme S1(a–c)†) and iron(III)-catalyst where FeCl3·6H2O or FeBr3 was used. It is interesting to find that the simply polymerization system not only showed typical features of living/controlled radical polymerization but also obtained well-defined PMMAs functionalized with moieties of phosphorus-containing ligands.
Experimental section
Materials
Methyl methacrylate (MMA) (+99%) was purchased from Shanghai Chemical Reagents Co. Ltd (Shanghai, China), which was removed inhibitor by passing through a neutral alumina column before use. Ethyl 2-bromoisobutyrate (EBiB) (98%) was purchased from Acros and used as received. Methyl-2-bromo-2-(bromomethyl)propanoate (MBBP, 1H NMR of C5H8Br2O2 (400 MHz, CDCl3): δ [ppm] = 1.99 (s, 3H, H-3); 3.69 (d, 2J = 9.8 Hz, 1H, CH2 at C2); 3.79 (s, 3H, OCH3 at C1); 4.19 (d, 2J = 9.8 Hz, 1H, CH2 at C2)) was synthesized as reported by literature.20 Bis(diphenylphosphino)methane (BDPPM, 98%), 1,2-bis(diphenylphosphino)ethane (BDPPE, 98%), triphenylphosphine oxide (TPPO, 98%), triphenylamine (TPA, 98%) tetra-n-butylphosphonium bromide (TBPBr, 99%), triphenylphosphine hydrobromide (TPP·HBr, 97%) were used as received from Sigma-Aldrich Co. Ltd. Triphenylphosphine (TPP, 99%, Sigma-Aldrich Co. Ltd) was recrystallized to constant melting point (m.p. 79.5–80 °C) from absolute alcohol. Tetra-n-butylammonium bromide (TBABr, 99%), iron(III) chloride hexahydrate (FeCl3·6H2O) (>99%) and ascorbic acid (AA) (+99.7%) were purchased from Shanghai Chemical Reagents Co. Ltd (Shanghai, China) and used as received. Solvents such as tetrahydrofuran (THF, +98%), toluene (+98%), anisole (+98%), N,N-dimethylformamide (DMF, +98%) and methanol (+98%) were used as received from Shanghai Chemical Reagents Co. All other chemicals were obtained from Shanghai Chemical Reagents Co. Ltd and used as received unless mentioned. The structures of some phosphorus-containing ligands used were listed in Scheme S1 (ESI†).General procedure for polymerization of MMA
A typical bulk polymerization procedure with the molar ratio of [MMA]0/[FeCl3·6H2O]0/[TPP]0 = 100/0.1/0.2 is as follows. A mixture was obtained by adding FeCl3·6H2O (5.2 mg, 0.018 mmol), TPP (10.0 mg, 0.038 mmol), MMA (2.0 mL, 18.8 mmol) to a dried ampoule with a stir bar. The mixture was thoroughly bubbled with argon for 20 min to eliminate the dissolved oxygen and then the ampoule was flame-sealed. The ampoule was transferred into an oil bath held by a thermostat at the desired temperature (90 °C) to polymerize under stirring. After the desired polymerization time, the ampoule was cooled by immersing into iced water. Afterwards, it was opened and the contents were dissolved in THF (∼2 mL), and precipitated into a large amount of methanol (∼200 mL). The polymer obtained by filtration was dried under vacuum until constant weight at 50 °C. The monomer conversion was determined gravimetrically.Characterization
The number-average molecular weight (Mn) and molecular weight distribution (Mw/Mn) of the resultant polymers were determined using a Waters 1515 gel permeation chromatograph (GPC) equipped with a refractive-index detector (Waters 2414), using HR 1 (pore size: 100 Å, 100–5000 Da), HR 2 (pore size: 500 Å, 500–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) and HR 4 (pore size 10
000 Da) and HR 4 (pore size 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å, 50–100
000 Å, 50–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) columns (7.8 × 300 mm, 5 μm beads size) with molecular weights ranging from 102–2 × 106 g mol−1. Tetrahydrofuran (THF) was used as the eluent at a flow rate of 1.0 mL min−1 and 30 °C. GPC samples were injected using a Waters 717 plus autosampler and calibrated with poly(methyl methacrylate) standards purchased from Waters. The 1H NMR and 31P NMR spectra of the obtained polymer were recorded on an INOVA 400 MHz nuclear magnetic resonance instrument using CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard.
000 Da) columns (7.8 × 300 mm, 5 μm beads size) with molecular weights ranging from 102–2 × 106 g mol−1. Tetrahydrofuran (THF) was used as the eluent at a flow rate of 1.0 mL min−1 and 30 °C. GPC samples were injected using a Waters 717 plus autosampler and calibrated with poly(methyl methacrylate) standards purchased from Waters. The 1H NMR and 31P NMR spectra of the obtained polymer were recorded on an INOVA 400 MHz nuclear magnetic resonance instrument using CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard.
Results and discussion
Effect of catalyst amount on bulk polymerization of MMA
The iron(III)-mediated bulk polymerization of MMA without any additional initiators was first investigated using TPP as the ligand under various molar ratios of [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [FeCl3·6H2O]0
[FeCl3·6H2O]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TPP]0. As shown in Table 1, the conversion of MMA decreased from 71.8% to 19.6% when the molar ratio of [MMA]0
[TPP]0. As shown in Table 1, the conversion of MMA decreased from 71.8% to 19.6% when the molar ratio of [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [FeCl3·6H2O]0
[FeCl3·6H2O]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TPP]0 decreasing from 100/1/2 to 100/0.1/0.2 after 3 h, which is attributed to the increasing amount of catalyst. At the same time, it can also be seen that molecular weight distributions (Mw/Mns) of the resultant PMMAs kept narrow. These results indicated that the polymerization of MMA can be successfully carried out by this simple catalyst system just comprising MMA, Fe(III) species and phosphorus ligand TPP.
[TPP]0 decreasing from 100/1/2 to 100/0.1/0.2 after 3 h, which is attributed to the increasing amount of catalyst. At the same time, it can also be seen that molecular weight distributions (Mw/Mns) of the resultant PMMAs kept narrow. These results indicated that the polymerization of MMA can be successfully carried out by this simple catalyst system just comprising MMA, Fe(III) species and phosphorus ligand TPP.
| Entry | Time [h] | [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Fe(III)]0 [Fe(III)]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ligand]0 [ligand]0 |
Conv. [%] | Mn,GPC [g mol−1] | Mw/Mn |
|---|---|---|---|---|---|
a Polymerization conditions: R = [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [FeCl3·6H2O]0 [FeCl3·6H2O]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TPP]0, VMMA = 2.0 mL, temperature = 90 °C. [TPP]0, VMMA = 2.0 mL, temperature = 90 °C. |
|||||
| 1 | 3.0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
71.8 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.06 |
| 2 | 3.0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
63.2 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.09 |
| 3 | 3.0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.33 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.67 0.67 |
42.4 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.10 |
| 4 | 3.0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 0.4 |
29.5 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.14 |
| 5 | 3.0 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
22.7 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.24 |
Generality of the simple catalyst system
In order to further test the generality of the catalyst system, the polymerizations of MMA were carried out under various polymerization conditions such as using FeBr3 instead of FeCl3·6H2O, in the presence of another two types of phosphorus ligands, bis(diphenylphosphino)methane (BDPPM) and 1,2-bis(diphenylphosphino)ethane (BDPPE), respectively. The effect of solvents (e.g., tetrahydrofuran (THF), anisole, toluene and DMF) on the polymerization was also investigated, the results were listed in Table 2. From entry 1 in Table 2, the monomer conversion and Mw/Mn is about 20.3% and 1.04 in 1.5 h, respectively, when FeBr3 was used as the catalyst. BDPPM and BDPPE were also qualified ligands for the MMA polymerization accompanying with low Mw/Mn values (entries 2 and 3 in Table 2). Furthermore, the polymerizations using TPP as ligand were carried out in tetrahydrofuran (THF), anisole, toluene and N,N-dimethylformamide (DMF), respectively, as shown in entries 4–7 in Table 2. It can be seen that all the polymerizations could be carried out in these four solvents and the Mw/Mn values of the obtained PMMAs were about 1.10, indicating that this simple catalyst system can be used in a wide range with a well-controlled polymerization process.| Entry | T [h] | [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Fe(III)]0 [Fe(III)]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ligand]0 [ligand]0 |
Solvent | Conv. [%] | Mn,GPC [g mol−1] | Mw/Mn |
|---|---|---|---|---|---|---|
| a Polymerization condition: VMMA = 2.0 mL, temperature = 90 °C. | ||||||
| 1 | 1.5 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1(FeBr3) 0.1(FeBr3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2(TPP) 0.2(TPP) |
Bulk | 20.3 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.04 |
| 2 | 2 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2(FeCl3·6H2O) 0.2(FeCl3·6H2O)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4(BDPPM) 0.4(BDPPM) |
Bulk | 31.9 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.14 |
| 3 | 2 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2(FeCl3·6H2O) 0.2(FeCl3·6H2O)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4(BDPPE) 0.4(BDPPE) |
Bulk | 32.1 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.10 |
| 4 | 4 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(FeCl3·6H2O) 1(FeCl3·6H2O)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2(TPP) 2(TPP) |
THF | 58.5 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.05 |
| 5 | 4 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(FeCl3·6H2O) 1(FeCl3·6H2O)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2(TPP) 2(TPP) |
Toluene | 36.7 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.15 |
| 6 | 4 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(FeCl3·6H2O) 1(FeCl3·6H2O)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2(TPP) 2(TPP) |
Anisole | 63.2 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.04 |
| 7 | 4 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(FeCl3·6H2O) 1(FeCl3·6H2O)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2(TPP) 2(TPP) |
DMF | 38.4 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.12 |
Polymerization kinetics
To further investigate the detailed polymerization behaviour, the polymerization kinetics of MMA was studied using FeCl3·6H2O and FeBr3 as the catalyst, repectively. Fig. 1(a) shows the kinetics of bulk polymerization of MMA with a molar ratio of [MMA]0/[Fe(III)]0/[TPP]0 = 100/0.1/0.2 at 90 °C. Both the polymerizations showed first order kinetics with respect to the monomer concentration, indicating that the number of active species remained constant during the polymerization process in two cases. By calculating the apparent rate constant of polymerization, kappp (Rp = −d[M]/dt = kp[Pn][M] = kappp [M]), as determined from the kinetic slopes, a kappp of 2.16 × 10−5 s−1 and 3.41 × 10−5 s−1 was obtained for FeCl3·6H2O and FeBr3 as the catalyst, respectively, which indicated that faster polymerization rate was observed in the presence of FeBr3. This is ascribed to the lower activation energy of C–Br bond than that of C–Cl as expected. Fig. 1(b) shows the evolution of the number-average molecular weight (Mn,GPC) values of the obtained PMMAs and molecular weight distribution (Mw/Mn) values on the conversion for the bulk polymerization of MMA. As shown in Fig. 1(b), the Mn,GPC values increased linearly with monomer conversion while keeping low Mw/Mn values (Mw/Mn = 1.09–1.24) in both cases, demonstrating the nature features of controlled/“living” radical polymerization.Another phosphorus-containing ligand BDPPM was also used to evaluate the polymerization kinetics of MMA in bulk under various catalyst concentrations at 90 °C. Analogous to Fig. 1(a), the polymerization kinetics shown in Fig. 2(a) was first order with respect to monomer concentration in both cases ([MMA]0/[FeCl3·6H2O]0/[BDPPM]0 = 100/0.2/0.4, 100/0.4/0.4). At the same time, it can be seen that the polymerization rate in the case of [MMA]0/[FeCl3·6H2O]0/[BDPPM]0 = 100/0.4/0.4 was faster than that of [MMA]0/[FeCl3·6H2O]0/[BDPPM]0 = 100/0.2/0.4. The corresponding kappp was 6.75 × 10−5 s−1 for 100/0.2/0.4, and 1.58 × 10−4 s−1 for 100/0.4/0.4, respectively, which is consistent with the results discussed above when using TPP as the ligand. It is specially mentioned that although no any thermal radical initiators and reducing agents this facile Fe(III)-mediated polymerization system showed faster polymerization rate. For example, a monomer conversion of 81.7% was obtained in 3 h with a molar ratio of [MMA]0/[FeCl3·6H2O]0/[BDPPM]0 = 100/0.4/0.4 at 90 °C. Fig. 2(b) shows the Mn,GPC values of the obtained PMMAs using BDPPM as the ligand increased linearly with monomer conversion while keeping low Mw/Mn values (Mw/Mn = 1.07–1.26). Therefore, all these results indicated that the iron(III)-mediated polymerization of MMA using BDPPM as the ligand in the absence of any additional initiators not only kept a higher polymerization rate but also didn't destroy the controlled/‘‘living’’ radical polymerization characteristics.
Analysis of chain end and discussion on mechanism
Firstly, in order to obtain more information about the mechanism of polymerization of MMA in the presence of FeCl3·6H2O/TPP, a radical scavenger (TEMPO) was added to the reaction system with [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [FeCl3·6H2O]0
[FeCl3·6H2O]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TPP]0
[TPP]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TEMPO]0 = 100
[TEMPO]0 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in bulk at 90 °C. Even after 24 h (entry 1 in Table 3), no polymers were obtained, which indicated that the polymerization was immediately terminated by the radical scavengers. Therefore, the iron(III)-mediated polymerization of MMA proceeded via a radical mechanism. Then, the polymerization of MMA was carried out in bulk at 90 °C, using FeCl3·6H2O/TPP as the complex and in the presence of a common ATRP initiator EBiB (AGET ATRP system) and thermal radical initiator azobis(isobutyronitrile) (AIBN) (reverse ATRP system), respectively. From entries 2 and 3 in Table 3, it can be seen that the polymerizations could be conducted with a controlled way as expected via ATRP mechanism.
1 in bulk at 90 °C. Even after 24 h (entry 1 in Table 3), no polymers were obtained, which indicated that the polymerization was immediately terminated by the radical scavengers. Therefore, the iron(III)-mediated polymerization of MMA proceeded via a radical mechanism. Then, the polymerization of MMA was carried out in bulk at 90 °C, using FeCl3·6H2O/TPP as the complex and in the presence of a common ATRP initiator EBiB (AGET ATRP system) and thermal radical initiator azobis(isobutyronitrile) (AIBN) (reverse ATRP system), respectively. From entries 2 and 3 in Table 3, it can be seen that the polymerizations could be conducted with a controlled way as expected via ATRP mechanism.
| Entry | T [h] | [ligand]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [additive]0 [additive]0 |
Conv. [%] | Mn,th [g mol−1] | Mn,GPC [g mol−1] | Mw/Mn |
|---|---|---|---|---|---|---|
a Polymerization conditions: [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Fe(III)]0 [Fe(III)]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ligand]0 = 100 [ligand]0 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, VMMA = 2.0 mL.b Temperature = 90 °C.c Temperature = 60 °C. 2, VMMA = 2.0 mL.b Temperature = 90 °C.c Temperature = 60 °C. |
||||||
| 1b | 24 | 2(TPP)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(TEMPO) 1(TEMPO) |
0 | NA | NA | NA |
| 2b | 1 | 2(TPP)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(EBiB) 1(EBiB) |
54.9 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.21 |
| 3c | 2 | 2(TPP)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5(AIBN) 0.5(AIBN) |
90.1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.25 |
| 4b | 16 | 2(TPPO)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0(no additives) 0(no additives) |
0 | NA | NA | NA |
| 5b | 16 | 2(TPPO)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(EBiB) 1(EBiB) |
0 | NA | NA | NA |
| 6b | 16 | 2(TPPO)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(EBiB) 1(EBiB)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2(AA) 2(AA) |
0 | NA | NA | NA |
However, where did the initial radicals come from to initiate the polymerization of MMA only in the presence of a phosphorus ligand and Fe(III) catalyst? Besides as the ligand, the possible functions of phosphorus-containing ligand (TPP) are shown in Scheme 1: (a) being oxidized to triphenylphosphine oxide (TPPO) in air (oxygen) or in the presence of trace dissolved O2 in the reaction mixture (Scheme 1(a)); (b) resulting in an ATRP initiator (1,2-dibromoisobutyrate) generated by the addition reaction between monomer MMA and FeCl3·6H2O (or FeBr3) as reported by Matyjaszewski et al.;17 (c) serving as a radical initiator as reported by Eldred and coworkers.19
To evaluate the possible multifunction of phosphorus ligand, TPPO was used as the phosphorus-containing ligand for the iron(III)-mediated polymerization of MMA. However, from entries 3–5 in Table 3, no polymers were obtained even though changing polymerization conditions, which suggested that TPPO was not a suitable ligand for iron(III) salts and TPPO could not act as an initiator to conduct the polymerization of MMA. In the reaction of Scheme 1(b), MMA reduced the higher-oxidation state metal to a lower one; at the same time, if methyl-2-chloro-2-(bromomethyl)propanoate (MCBP) generated in the case of FeCl3·6H2O or methyl-2-bromo-2-(bromomethyl)propanoate (MBBP) produced in the presence of FeBr3 can serve as an ATRP initiator, then the mechanism of polymerization would comply with a normal ATRP in the presence of the occurrence of the FeCl2/FeCl3/TPP catalyst system. The key point is whether the MCBP or MBBP generated in situ can be used as an effective ATRP initiator. In order to confirm this issue, MBBP was synthesized solely and used as an ATRP initiator to conduct ATRP of MMA. The results are shown in Table 4. It is very surprising to find that, as shown in entry 1 of Table 4, no polymers were obtained even after 70 h at 90 °C when MBBP was used as the initiator solely. Contrarily, when a common ATRP initiator EBiB was used as the initiator solely (other polymerization conditions the same, entry 7 in Table 4), a 54.9% of monomer conversion could be available in 1 h. In addition, from entries 2–6 in Table 4, it can be concluded that the more the amounts of MBBP used ([MBBP]0/[EBiB]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0.1
1 to 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) the slower the rate for MMA polymerization when dual initiators MBBP and EBiB were used to conduct the polymerizations of MMA, indicating that MBBP was not an active initiator or an inhibitor in this case at least. Therefore, these results combination with the polymerization kinetics data mentioned above demonstrated that the possible reactions of TPP shown in Schemes 1(a) and (b) may be ignored in this polymerization system.
1) the slower the rate for MMA polymerization when dual initiators MBBP and EBiB were used to conduct the polymerizations of MMA, indicating that MBBP was not an active initiator or an inhibitor in this case at least. Therefore, these results combination with the polymerization kinetics data mentioned above demonstrated that the possible reactions of TPP shown in Schemes 1(a) and (b) may be ignored in this polymerization system.
| Entry | T [h] | [MMA]0 [MBBP]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [EBiB]0 [EBiB]0 |
Conv. [%] | Mn,GPC [g mol−1] | Mw/Mn |
|---|---|---|---|---|---|
a Polymerization conditions: [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [FeCl3·6H2O]0 [FeCl3·6H2O]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TPP]0 = 100 [TPP]0 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; VMMA = 2.0 mL, temperature = 90 °C. 2; VMMA = 2.0 mL, temperature = 90 °C. |
|||||
| 1 | 70 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0 | NA | NA |
| 2 | 70 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | NA | NA |
| 3 | 70 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | NA | NA |
| 4 | 70 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.33 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
21.6 | 9200 | 1.19 |
| 5 | 3 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40.7 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.18 |
| 6 | 1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
45.5 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.17 |
| 7 | 1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
54.9 | 5500 | 1.21 |
The novel photocatalyst agents21 highlight the development of a highly responsive controlled/“living” radical polymerization under UVA. For example, in the presence of bis(diphenylphosphino)methane (BDPPM) (also used in this manuscript), photoinduced-ATRP of methacrylates and acrylates initiated by ethyl α-bromophenylacetate (EBPA) in the presence of a dinuclear gold(I) complex based photocatalyst [Au2(BDPPM)2]Cl2 has been reported in solution and in laminate under UVA and visible-light photoreductive conditions.22 Actually, as reported by Eldred and coworkers TPP can initiate polymerization of MMA under ultraviolet light or heating conditions.19 The mechanism is showed in Scheme 1(C). The successfully initiating the polymerization of MMA was confirmed by our experiment just using TPP as the initiator with molar ratio of [MMA]0/[TPP]0 = 100/2 in bulk at 90 °C (entry 2 in Table 5). The obtained PMMA was analyzed by 31P NMR spectroscopy. The chemical shift by 31P NMR at δ = 25.0 ppm (ESI, Fig. S1†) was corresponded to the P protons in the chain end of PMMA. Meanwhile, the results of LC-MS confirmed the formation (a peak observed at m/z = 363.15, ESI Fig. S2†) of TPP–MMA complex (as shown in Scheme 1(C)) when mixing MMA and TPP with a molar ratio of 10/1, which can result in TPP-initiated polymerization of MMA. In addition, TPP was also used as a thermal radical initiator to initiate RAFT polymerization of MMA with 2-cyanoprop-2-yl 1-dithionaphthalate (CPDN) as the RAFT agent. Although the polymerization rate is slow (27.8% of monomer conversion in 30 h) while Mn,GPC (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1) value was close to the theoretical one (Mn,th = 13
300 g mol−1) value was close to the theoretical one (Mn,th = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1) and low Mw/Mn value (Mw/Mn = 1.11) was kept (entry 3 in Table 5). All these results proved that TPP can initiate the polymerization of MMA.
900 g mol−1) and low Mw/Mn value (Mw/Mn = 1.11) was kept (entry 3 in Table 5). All these results proved that TPP can initiate the polymerization of MMA.
| Entry | T [h] | Components | Conv. [%] | Mn,GPC [g mol−1] | Mw/Mn |
|---|---|---|---|---|---|
a Polymerization conditions: VMMA = 2.0 mL, temperature = 90 °C.b CTA = CPDN (2-cyanoprop-2-yl 1-dithionaphthalate), Mn,th = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1. 900 g mol−1. |
|||||
| 1 | 70 | [MMA]0, bulk | 0 | NA | NA |
| 2 | 70 | [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TPP]0 = 100 [TPP]0 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
8.6 | 880![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.22 |
| 3b | 30 | [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CTA]0 [CTA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TPP]0 = 100 [TPP]0 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
27.8 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.11 |
| 4 | 70 | [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TPA]0 = 100 [TPA]0 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0 | NA | NA |
| 5 | 16 | [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [FeCl3·6H2O]0 [FeCl3·6H2O]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBPBr]0 = 100 [TBPBr]0 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0 | NA | NA |
| 6 | 16 | [MMA]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [FeCl3·6H2O]0 [FeCl3·6H2O]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TPP·HBr]0 = 100 [TPP·HBr]0 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0 | NA | NA |
It is noted that triphenylamine (TPA) with a similar structure of TPP could not initiate the polymerization of MMA (entry 4 in Table 5). This can be explained by the fact that the nitrogen atom, unlike phosphorus, does not have any d orbital, and therefore it is difficult to participate in the complex formation with MMA. Furthermore, tetra-n-butylphosphonium bromide (TBPBr) and triphenylphosphine hydrobromide (TPP·HBr) were used as the ligands for the iron(III)-mediated polymerization of MMA; however, no polymers were obtained even after 16 h (entries 5 and 6 in Table 5). This is ascribed to that ionic phosphorus compounds could not participate in the complex formation with MMA.
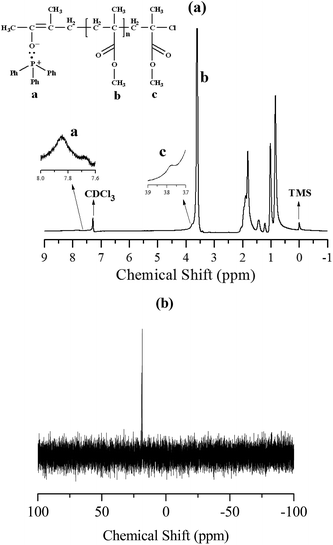
As discussed above, TPP could initiate conventional radical polymerization of MMA. A well-controlled polymerization process was obtained because of the occurrence of I/FeX3/TPP catalyst system, which I came from TPP-initiated polymerization.23 Therefore, the plausible mechanism for this facile polymerization system comprising MMA/FeX3/TPP may be consistent with that of reverse ATRP. As shown in Scheme 2, in the initiation step, once generated from TPP and MMA, the initiating radicals or the propagating radicals, I or I–P1, can abstract the halogen atom X from the oxidized transition-metal species, XMtn+1/L, to form the reduced transition-metal species, Mtn, and the dormant species, I–X or I–P1–X. In the subsequent steps, the transition-metal species, Mtn/L, promotes exactly the same ATRP process as normal ATRP where R–X/Mtn/L are used as the initiation system. Instead of first activation of a dormant species, R–X, with Mtn/L, as in the case of normal ATRP, reverse ATRP originates from the deactivation reaction between radicals, I or I–P1, and XMtn+1/L. In order to further certify the mechanism, the chain end of the obtained PMMA was analyzed by 1H NMR and 31P NMR spectroscopy, as shown in Fig. 3. In Fig. 3(a), the chemical shifts at δ = 7.7–8.0 ppm (a in Fig. 3(a)) were attributed to the aromatic protons from the TPP and MMA complex moieties. A signal at 3.79 ppm (c in Fig. 3(a)) was corresponded to the protons of the methyl ester group at the chain end as mentioned by Sawamoto.3a In Fig. 3(b), the chemical shifts at δ = 18.4–19.0 ppm was corresponded to the P protons in the chain end of PMMA.
 | ||
| Scheme 2 The plausible polymerization mechanism for iron(III)-mediated polymerization of MMA in the presence of iron(III) catalyst and phosphorus-containing ligands without any additional initiators. | ||
Conclusions
A simple polymerization system involving of MMA, FeX3 (X = Cl, Br) and phosphorus ligands (TPP, BDPPM, BDPPE) was successfully established in a controlled fashion. The possible polymerization mechanism may be consistent with reverse ATRP, in which phosphorus-containing reagents acted as both ligand and thermal radical initiator.Acknowledgements
The financial was supported by the National Natural Science Foundation of China (Nos 21404051 and 21404052), the Natural Science Foundation of Shandong Province (Nos ZR2014BQ016 and BS2014CL040), the Talent Introduction Special Funds of Ludong University (Nos 2014012 and 2014017), the Program for Scientific research Innovation Team in Colleges and universities of Shandong Province and Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application (Soochow University).References and Notes
- (a) M. Kato, M. Kamigaito, M. Sawamoto and T. Higashimura, Macromolecules, 1995, 28, 1721 CrossRef CAS; (b) J. S. Wang and K. Matyjaszewski, J. Am. Chem. Soc., 1995, 117, 5614 CrossRef CAS; (c) M. Kamigaito, T. Ando and M. Sawamoto, Chem. Rev., 2001, 101, 3689 CrossRef CAS PubMed; (d) K. Matyjaszewski and J. H. Xia, Chem. Rev., 2001, 101, 2921 CrossRef CAS PubMed; (e) M. Ouchi, T. Terashima and M. Sawamoto, Chem. Rev., 2009, 109, 4963 CrossRef CAS PubMed; (f) L. J. Bai, W. X. Wang, H. Chen, M. H. Wang and Z. P. Cheng, RSC Adv., 2015, 5, 34769 RSC; (g) Y. Y. Xu, J. M. Sun, H. Chen and L. J. Bai, RSC Adv., 2015, 5, 58393 RSC; (h) Y. Y. Xu, J. M. Sun, H. Chen and L. J. Bai, RSC Adv., 2015, 5, 37780 RSC.
- (a) G. H. Zhu, L. F. Zhang, X. Q. Pan, W. Zhang, Z. P. Cheng and X. L. Zhu, Macromol. Rapid Commun., 2012, 33, 2121 CrossRef CAS PubMed; (b) T. Pintauer and K. Matyjaszewski, Chem. Soc. Rev., 2008, 37, 1087 RSC; (c) T. Pintauer and K. Matyjaszewski, Coord. Chem. Rev., 2005, 249, 1155 CrossRef CAS PubMed.
- (a) T. Ando, M. Kamigaito and M. Sawamoto, Macromolecules, 1997, 30, 4507 CrossRef CAS; (b) K. Matyjaszewski, M. Wei, J. Xia and N. E. McDermott, Macromolecules, 1997, 30, 8161 CrossRef CAS; (c) Y. Louie and R. H. Grubbs, Chem. Commun., 2000, 1479 RSC; (d) M. Ishio, T. Terashima, M. Ouchi and M. Sawamoto, Macromolecules, 2010, 43, 920 CrossRef CAS; (e) R. K. ÓReilly, V. C. Gibson, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 2003, 125, 8450 CrossRef PubMed; (f) S. Niibayashi, H. Hayakawa, R.-H. Jin and H. Nagashima, Chem. Commun., 2007, 1855 RSC; (g) C. Uchiike, T. Terashima, M. Ouchi, T. Ando, M. Kamigaito and M. Sawamoto, Macromolecules, 2007, 40, 8658 CrossRef CAS; (h) L. F. Zhang, Z. P. Cheng, F. Tang, Q. Li and X. L. Zhu, Macromol. Chem. Phys., 2008, 209, 1705 CrossRef CAS PubMed; (i) L. J. Bai, L. F. Zhang, J. Zhu, Z. P. Cheng and X. L. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2002 CrossRef CAS PubMed; (j) L. F. Zhang, Z. P. Cheng, Y. T. Lü and X. L. Zhu, Macromol. Rapid Commun., 2009, 30, 543 CrossRef CAS PubMed; (k) L. F. Zhang, Z. P. Cheng, S. P. Shi, Q. Li and X. L. Zhu, Polymer, 2008, 49, 3054 CrossRef CAS PubMed; (l) Z. J. Deng, L. H. Qiu, L. J. Bai, Y. X. Zhou, B. C. Lin, J. Zhao, Z. P. Cheng, X. L. Zhu and F. Yan, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1605 CrossRef CAS PubMed; (m) Z. J. Deng, J. N. Guo, L. H. Qiu, Y. X. Zhou, L. Xia and F. Yan, Polym. Chem., 2012, 3, 2436 RSC; (n) Z. J. Deng, J. N. Guo, L. H. Qiu, C. Yuan, Y. X. Zhou and F. Yan, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 664 CrossRef CAS PubMed.
- (a) T. Ando, M. Kato, M. Kamigaito and M. Sawamoto, Macromolecules, 1996, 29, 1070 CrossRef CAS; (b) H. Uegaki, Y. Kotani, M. Kamigaito and M. Sawamoto, Macromolecules, 1998, 31, 6756 CrossRef CAS; (c) M. Ouchi, H. Yoda, T. Terashima and M. Sawamoto, Polym. J., 2012, 44, 51 CrossRef CAS PubMed; (d) D. He, S. K. Noh and W. S. Lyoo, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 4594 CrossRef CAS PubMed.
- (a) C. Granel, P. Dubois, R. Jerome and P. Teyssie, Macromolecules, 1996, 29, 8576 CrossRef CAS; (b) S. Pascual, B. Coutin, M. Tardi, A. Polton and J. P. Varion, Macromolecules, 1999, 32, 1432 CrossRef CAS.
- M. Ishio, M. Katsube, M. Ouchi, M. Sawamoto and Y. Inoue, Macromolecules, 2009, 42, 188 CrossRef CAS.
- (a) F. D. Lena and K. Matyjaszewski, Prog. Polym. Sci., 2010, 34, 959 CrossRef PubMed; (b) L. J. Bai, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Polym. Chem., 2012, 3, 2685 RSC.
- K. Min, W. Jakubowski and K. Matyjaszewski, Macromol. Rapid Commun., 2006, 27, 594 CrossRef CAS PubMed.
- J. S. Wang and K. Matyjaszewski, Macromolecules, 1995, 28, 7572 CrossRef CAS.
- (a) J. Gromada and K. Matyjaszewski, Macromolecules, 2001, 34, 7664 CrossRef CAS; (b) M. Li, K. Min and K. Matyjaszewski, Macromolecules, 2004, 37, 2106 CrossRef CAS; (c) M. Li, N. M. Jahed, K. Min and K. Matyjaszewski, Macromolecules, 2004, 37, 2434 CrossRef CAS.
- (a) K. Matyjaszewski, W. Jakubowski, K. Min, W. Tang, J. Y. Huang, W. A. Braunecker and N. V. Tsarevsky, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15309 CrossRef CAS PubMed; (b) L. Mueller, W. Jakubowski, W. Tang and K. Matyjaszewski, Macromolecules, 2007, 40, 6464 CrossRef CAS; (c) L. F. Zhang, J. Miao, Z. P. Cheng and X. L. Zhu, Macromol. Rapid Commun., 2010, 31, 275 CrossRef CAS PubMed; (d) G. H. Zhu, L. F. Zhang, Z. B. Zhang, J. Zhu, Y. F. Tu, Z. P. Cheng and X. L. Zhu, Macromolecules, 2011, 44, 3233 CrossRef CAS; (e) D. Konkolewicz, A. J. D. Magenau, S. E. Averick, A. Simakova, H. K. He and K. Matyjaszewski, Macromolecules, 2012, 45, 4461 CrossRef CAS; (f) Y. P. Borguet and N. V. Tsarevsky, Polym. Chem., 2012, 3, 2487 RSC; (g) X. H. Liu, J. Wang, F. J. Zhang, S. L. An, Y. L. Ren, Y. H. Yu, P. Chen and S. Xie, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4358 CrossRef CAS PubMed; (h) A. Nese, Y. C. Li, S. S. Sheiko and K. Matyjaszewski, ACS Macro Lett., 2012, 1, 991 CrossRef CAS; (i) K. Mukumoto, Y. Wang and K. Matyjaszewski, ACS Macro Lett., 2012, 1, 599 CrossRef CAS.
- (a) W. Jakubowski and K. Matyjaszewski, Macromolecules, 2005, 38, 4139 CrossRef CAS; (b) K. Min, H. F. Gao and K. Matyjaszewski, J. Am. Chem. Soc., 2005, 127, 3825 CrossRef CAS PubMed; (c) J. K. Oh, H. C. Dong, R. Zhang, K. Matyjaszewski and H. Schlaad, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4764 CrossRef CAS PubMed; (d) J. Miao, W. W. He, L. F. Zhang, Y. Wang, Z. P. Cheng and X. L. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2194 CrossRef CAS PubMed; (e) H. J. Jiang, L. F. Zhang, J. L. Pan, X. W. Jiang, Z. P. Cheng and X. L. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2244 CrossRef CAS PubMed; (f) Y. X. Wang, X. L. Li, F. F. Du, H. S. Yu, B. K. Jin and R. K. Bai, Chem. Commun., 2012, 48, 2800 RSC.
- (a) W. Jakubowski, K. Min and K. Matyjaszewski, Macromolecules, 2006, 39, 39 CrossRef CAS; (b) R. Nicolay, Y. Kwak and K. Matyjaszewski, Angew. Chem., Int. Ed., 2010, 122, 551 CrossRef PubMed.
- C. R. Becer, R. Hoogenboom, D. Fournier and U. S. Schubert, Macromol. Rapid Commun., 2007, 28, 1161 CrossRef CAS PubMed.
- (a) Z. G. Xue, D. He, S. K. Noh and W. S. Lyoo, Macromolecules, 2009, 42, 2949 CrossRef CAS; (b) Z. G. Xue, H. S. Oh, S. K. Noh and W. S. Lyoo, Macromol. Rapid Commun., 2008, 29, 1887 CrossRef CAS PubMed; (c) Z. G. Xue, N. T. B. Linh, S. K. Noh and W. S. Lyoo, Angew. Chem., Int. Ed., 2008, 47, 6426 CrossRef CAS PubMed; (d) Z. G. Xue, B. W. Lee, S. K. Noh and W. S. Lyoo, Macromol. Rapid Commun., 2008, 29, 1887 CrossRef CAS PubMed; (e) D. He, Z. G. Xue, M. Y. Khan, S. K. Noh and W. S. Lyoo, Polymer, 2007, 48, 4704 CrossRef PubMed; (f) X. X. Chen, M. Y. Khan and S. K. Noh, Polym. Chem., 2012, 3, 1971 RSC.
- A. E. Acar, M. B. Yağci and L. J. Mathias, Macromolecules, 2000, 33, 7700 CrossRef CAS.
- A. K. Nanda, S. C. Hong and K. Matyjaszewski, Macromol. Chem. Phys., 2003, 204, 1151 CrossRef CAS PubMed.
- Z. P. Cheng, X. L. Zhu, N. C. Zhou and J. M. Lu, J. Appl. Polym. Sci., 2003, 90, 1532 CrossRef CAS PubMed.
- T. J. Mao and R. J. Eldred, J. Polym. Sci., Part A-1: Polym. Chem., 1967, 5, 1741 CrossRef CAS PubMed.
- S. Werle, T. Fey, J. M. Neudörfl and H. G. Schmalz, Org. Lett., 2007, 9, 3555 CrossRef CAS PubMed.
- (a) J. F. Quinn, L. Barner, C. Barner-Kowollik, E. Rizzardo and T. P. Davis, Macromolecules, 2002, 35, 7620 CrossRef CAS; (b) J. C. Liu, I. Y. Rad, F. Sun and J. W. Stansbury, Polym. Chem., 2014, 5, 227 RSC; (c) H. Hua, Y. Xiong, C. Fu and N. Li, RSC Adv., 2014, 74, 39273 RSC.
- F. Nzulu, S. Telitel, F. Stoffelbach, G. Bernadette, F. Morlet-Savary, J. Lalevée, L. Fensterbank, J. P. Goddard and C. Ollivier, Polym. Chem., 2015, 6, 4605 RSC.
- (a) J. H. Xia and K. Matyjaszewski, Macromolecules, 1997, 30, 7692 CrossRef CAS; (b) Z. P. Cheng, X. L. Zhu, L. F. Zhang, N. C. Zhou and X. R. Xue, Polym. Bull., 2003, 49, 363 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra10317g |
| This journal is © The Royal Society of Chemistry 2015 |