Microwave-assisted and large-scale synthesis of SnO2/carbon-nanotube hybrids with high lithium storage capacity
Junting Zhang,
Youqi Zhu,
Chuanbao Cao* and
Faheem K. Butt
Research Center of Materials Science, Beijing Institute of Technology, Beijing 100081, China. E-mail: cbcao@bit.edu.cn; Fax: +86 10 68912001; Tel: +86 10 68913792
First published on 29th June 2015
Abstract
A novel SnO2/carbon-nanotube hybrid has been successfully synthesized at large scale by an ultrafast and environmentally benign microwave-assisted technique under atmospheric condition. The as-synthesized hybrids feature a unique structure with ultrathin SnO2 nanosheets and nanoparticles composited on multiwalled carbon nanotubes (CNTs). The specific surface area of the hybrids is as high as 145.46 m2 g−1 even with high SnO2 loading percentage of ∼85 wt%. The SnO2/carbon-nanotube hybrids used as anode material for LIB exhibit an ultrahigh initial capacity of 3247.5 mA h g−1 with a large reversible capacity of 1616.5 mA h g−1 at 200 mA g−1. After 80 repeated discharge and charge cycles, a reversible capacity of 710.4 mA h g−1 is maintained. As Compared to pure SnO2 nanosheets, the SnO2/carbon-nanotube hybrids exhibit enhanced electrochemical lithium ion storage, which could be ascribed to the unique architecture with more active sites and favorable channel for Li+ insertion/extraction. Hence, this microwave-assisted method demonstrates a high-efficiency and timesaving way to synthesize advanced SnO2-based anode materials for promising scalable commercialization.
1. Introduction
Lithium-ion batteries (LIBs) have become the predominant energy storage system for portable electronic devices and have demonstrated a great potential in large scale technological applications in electrical vehicles, owing to their advantages of high energy density, long lifespan, and environmental benignity.1 The electrode materials have critical role in the performance of a LIB.2 As for Li-insertion mechanism the capacity of the currently available carbon-based anodes materials is limited to 372 mA h g−1. This value of capacity cannot meet the requirements of the aforemention applications.3 In this respect, tin oxide (SnO2) has been proposed as promising alternative anode materials for their relative abundance, safe lithiation-insertion potential and high theoretical specific capacity of 782 mA h g−1, which can provide twice as more capacity than the commonly used graphite-based anodes for LIB.4 However, the bottlenecks in practical utilization of tin dioxide are rapid capacity fading and poor cycling stability arising from the large specific volume expansion (∼250%) in the discharging and charging processes. The processes of Li–Sn alloying and Li–Sn dealloying can cause stress-induced material malfunction, which create the loss of electrical contact within the anode material.5 Nevertheless, diverse tactics have been projected to mitigate the above-mentioned setbacks of SnO2-based anodes. One of the effectual method is to synthesize versatile types of SnO2 nanostructures such as nanospheres, nanotubes, nanosheets, and hollow nanostructures6 that can sustain the volumetric expansion.1 However, pure SnO2 anode always suffers from native small charge/ionic conductivity and self-aggregation between nanoparticles resulting in relatively low practical capacity, which are still unsatisfactory for high power density demands.6 For these reasons, building hybrid nanostructures has been considered as an effective approach toward fabricating high-performance electrodes in LIBs. The performance of metal oxides for Li-ion battery or other energy storage system can be enhanced by carbon nanomaterials. For example, Y. Z. Su and his coworkers fabricated a two-dimensional (2D) core–shell nanostructure within carbon layers to improve the electrochemical performance of metal oxides in lithium storage.7 Meanwhile, S. Li et al. demonstrate an strategy for the controlled growth of MO/MH (such as Co3O4, Fe2O3, and Ni(OH)2) NPs on graphene to construct unique 2D nanohybrids, which deliver excellent rate capability and cycle performance when used as electrode materials for supercapacitors.8 Moreover, various carbon materials have been composited with metal oxides for better electrochemical performance.9–12 Particularly, the incorporation of SnO2 with carbonaceous conductive matrix, such as graphene and CNTs, has received great attention and is expected to result in an improved lithium storage performance.13–15 For example, Yang and co-workers synthesized graphene-based SnO2 composite under a hydrothermal condition, which maintained a reversible capacity of 621.5 mA h g−1 at a current density of 782 mA g−1.16Carbon nanotubes (CNTs) have long been regarded as desirable one dimensional nanomaterials to functionalize other materials for application in energy storage, because of their unique 1D tubular geometrical structure, high electrical conductivity, and large surface area.17 When constructed into electrode materials, the contained CNTs can form a three-dimensional electric network and strongly restrain the volume expansion or pulverization of electrochemical active materials.18 To date, there have been many reports about the synthesis of this composites, of which the hydrothermal method is the most widely used.19–21 Ding et al. prepared multiwalled carbon nanotubes coated with SnO2 nanoparticles with the hydrothermal method which exhibit the charge capacity of 608 mA h g−1 and discharge capacity of 646 mA h g−1 in the 10th cycle.19 Wen and co-workers also synthesized mesoporous tin oxide (SnO2) overlaying on the surface of MWCNTs using the same method, however, the capacity decreases dramatically to 344.5 mA h g−1 after 50 cycles.22 This process usually takes more than 12 h and maintains at over 160 °C, leading to a low productivity, thus hinders its commercialization. Therefore, it is a challenge to develop a cost-effective, time saving, and simple method with high-yield that has commercial prospects.
Recently, our research group has proposed a microwave-assisted method to synthesize 2D ultrathin metal oxide nanosheets.23–27 The designed scheme is distinctive in its capability to be scaled-up without suffering from the thermal gradient effects, advantages of faster reaction time, thus enabling a potentially industrially significant improvement in nanomaterial synthetic methods over conventionally used methods.28 Herein, the SnO2/carbon nanotube hybrids have been effectively synthesized via a microwave-assisted technique. We combine the microwave irradiation and structure directing of CTAB and HMT to synthesize and stabilize SnO2/carbon nanotube hybrids. HMT is the alkaline reagents to stabilize the hybrids and CTAB is the surfactant to regulate the crystal growth behavior.23 The reaction time for fabrication of SnO2 has been drastically shortened to 60 minutes which implies the economical, green and energy conserved type of reactor design. The main feature of the reactor system is that it is open to air without any use of high pressure vessels. This reaction system requires much simpler condition than traditional methods. Moreover, by using this technique, we can get the expected product at larger amount up to gram-scale at one time compared to the hydrothermal method, making it scalable for commercialization. The obtained SnO2/carbon nanotube hybrids deliver notably enhanced performances over the pure SnO2 nanosheets and carbon nanotubes.
2. Experimental details
2.1 Materials synthesis
The microwave-assisted synthesis procedure of SnO2/carbon-nanotube hybrids is as follows: 2.1662 g SnCl2·2H2O, 1.3995 g CTAB (hexadecyltrimethylammonium), and 1.3458 g HMT (hexamethylenetetramine) were dissolved into 240 mL deionized H2O. Then 0.2439 g carbon nanotubes were dispersed into the above solution under ultrasonication for 20 minutes. The obtained mixture was shifted into a 1000 mL round-bottom flask after stirring for 2 hours. The mixture was then used for the microwave-assisted synthesis. A SINEO MAS-II microwave reactor (Sineo Microwave Chemistry Technology Co., LTD, Shanghai China) was employed to provide microwave irradiation. The microwave heating power was 700 W and heating time was 60 minutes. The solution was heated from room temperature to 93 °C in only 2 minutes under ambient condition in microwave reaction system. The microwave system was attached with a refluxing system in order to maintain the vapors in the reaction system. Thus the water could be retained and the temperature was stable around 93 °C. After cooling down to room temperature, the black precipitate was collected by centrifugation, washed thoroughly with deionized water and ethanol, and then dried in vacuum at 100 °C overnight. The final product was calcined at 500 °C for 2 h in argon gas to remove the residual organic molecule.2.2 Structure characterization
The crystallographic information of the prepared samples was collected using X-ray diffraction (XRD, Bruker D8) using Cu Kα (λ = 0.15418 nm) radiation. The product morphology was examined by transmission electron microscopy (TEM; JEM-2100, 200 kV accelerating voltage). X-ray photoelectron spectra (XPS, PHI Quanteral II, Japan) was used to determine the composition and electronic state of the SnO2/carbon-nanotube hybrids. The Brunauer–Emmett–Teller specific surface area and porosity was evaluated using NOVA4200e nitrogen adsorption instrument (Quantachrome Instruments, USA). Thermogravimetric analysis (TGA) was performed on a Q50 thermoanalyzer with air as the carrier gas at a heating rate of 10 °C min−1.2.3 Electrochemical measurements
The electrochemical charge and discharge measurements were carried out under ambient temperature using two-electrode coin cells (CR 2025-type) with high-purity lithium foil which serves as both counter and reference electrodes. The working electrode consisted of 78 wt% of the prepared samples, 12 wt% of acetylene black, and 10 wt% of PVDF (polyvinylidene difluoride) binder. The material was dissolved in 1-methyl-2-pyrrolidinone (NMP) to form slurry and was coated on copper foil. After drying under vacuum at 80 °C for 12 h and compressing at 1.0 × 106 Pa for 30 s, the Cu foil were punched into 14 mm diameter disks. Then coin cells were mounted up in an argon-filled glove box (Universal 2440/750, Mikrouna (China) Co., Ltd.) with concentrations of moisture and oxygen below 1.0 ppm. The measurements on electrochemical half cells were carried out on CT2001A Land battery testing systems (Hannuo Electronics Co., Ltd. China) and IM6e electrochemical workstation (Zahner, Germany).3. Results and discussion
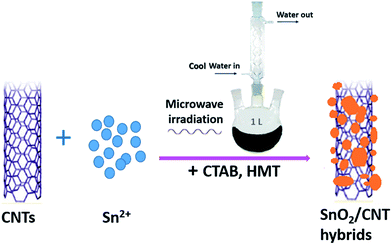
The process of the fabrication of the as-prepared SnO2/carbon nanotube hybrids was illustrated in Scheme 1. The SnCl2·2H2O and carbon nanotubes (CNTs) were added into deionized water and shifted into a round-bottom flask. The synthesis system was fixed with a refluxing system to retain the water. With the combination of microwave irradiation and the structure directing of CTAB and HMT, we successfully synthesized SnO2/carbon nanotube hybrids.Fig. 1a illustrates the X-ray diffraction peaks of the SnO2/carbon nanotube hybrids. The peaks can be readily indexed as a tetragonal rutile SnO2 (JCPDF card no. 41-1445; space group: P42/mnm; ao = 4.738 Å, co = 3.1865 Å). A high crystallinity can be suggested by the strong and sharp peaks. The inset in Fig. 1a shows the photograph of the final product, which is weighed to be 1.53 g. Fig. 1b shows the thermogravimetric analysis of the as-synthesized SnO2/carbon nanotube hybrids under air atmosphere to determine the percentage of SnO2 nanosheets and nanoparticles in the nanocomposites. About 15% of weight loss recorded from 400 to 650 °C is attributed to the decomposition of the amorphous CNTs, indicating the content of SnO2 nanosheets and nanoparticles is about 85 wt%, which indicates that it would be favorable for the increase in capacity of the hetero-structures, SnO2/CNTs, as an anode for LIBs. Fig. 1c presents a transmission electron microscopy (TEM) image of the product, which exhibits that CNTs formed a composite with SnO2 ultrafine nanosheets and nanoparticles. The corresponding selected area electron diffraction (SAED) pattern (inset of Fig. 1c) exhibits three intense diffraction rings indexed to the (110), (101), (211) planes of tetragonal rutile-like SnO2, which proves that the SnO2 around CNTs is polycrystalline in nature.29 Further high-resolution TEM image (HRTEM, Fig. 1d) provides a close view of the ultrafine SnO2 nanosheet grown on the surface of CNT. It can be observed that the SnO2 nanosheets and nanoparticles attached on CNTs' surface firmly, where the diameter of the CNT is about 20 nm. The marked interplanar spacing of 3.38 Å and 2.69 Å correspond to the (110) and (101) plane of pure SnO2, respectively. The tight contact between the SnO2 nanosheets and nanoparticles and the CNTs can provide an excellent conductive network to enable the electron transfer during the lithium alloying and dealloying process.30 The high mechanical flexibility of CNTs can act as a buffer to evade the volume expansion and contraction of SnO2 during Li+ insertion and extraction, which would direct to desirable cycle capability of SnO2/carbon nanotube hybrids.31
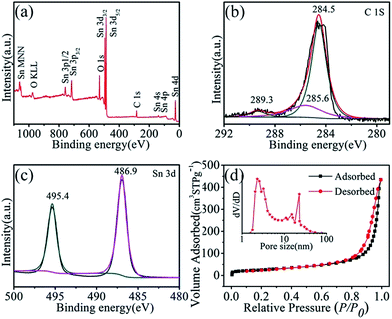
X-ray photoelectron spectroscopy (XPS) analyzed the surface composition and chemical states of the species in the SnO2/CNT hybrids. The wide survey XPS spectrum (Fig. 2a) reveals the presence of Sn, O and C, no other elements were detected. Fig. 2b displays the fine spectra of the C 1s region of SnO2/CNT hybrids samples, which can be further divided into three peaks. The strong C1s peak at 284.5 eV corresponds to C–C bonds in carbon nanotubes. The two weaker peaks located at 285.6 eV and 289.3 eV are related to carbon in epoxide C–O group and carboxyl carbon O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.32,33 These C–O covalent bonds are result of the synergetic effect between the interface of SnO2 and carbon nanotubes, which not only perk up the structural firmness but also compose the hybrid structure more conductive as compared to the semiconducting SnO2.30 In Sn 3d spectrum (Fig. 2c), two symmetrical peaks at 486.9 eV and 495.4 eV are attributed to Sn 3d5/2 and Sn 3d3/2, respectively, which confirmed the formation of SnO2.34,35 To investigate the surface area and pore size distribution of the SnO2/CNT hybrids, Brunauer–Emmett–Teller (BET) method was carried out with a nitrogen adsorption–desorption process, as shown in Fig. 2d. The N2 adsorption–desorption isotherm demonstrates a typical type IV nitrogen adsorption branch with an H3 hysteresis loop, which indicates the porous structure,36,37 leading to a high BET surface area of 145.46 m2 g−1 with total pore volume of 0.6957 cm3 g−1. The pore size distribution was carried out by Barrett–Joyner–Halenda model, as depicted in the inset of Fig. 2d. The two peaks represent that the average pore sizes of SnO2 and CNT are measured to be 2.3 nm and 22.76 nm, respectively, which is consistent with the result of the TEM observation. It is proved by the experiments that a high BET surface area could be advantageous to the electrochemical performance of lithium ion batteries. Y. Dong et al. fabricated SnO2 nanoparticles fixed on graphene nanosheets with a high (BET) surface area of 295 m2 g−1, which exhibits good cyclic performance for lithium ion batteries.6 J. Chen and his coworkers prepared SnO2/Carbon composite having a high (BET) surface area of 375 m2 g−1, resulting in a high reversible average capacity used as anodes.44 Moreover, Y. P. Zhai et al. confirmed that the capacitance is in direct proportion to the electrode surface area according to a formula in their review.38 Compared with pure SnO2 nanosheets,23 SnO2/CNT hybrids have an increase in both surface area and pore size as expected. Generally speaking, the superhigh BET surface area value is not desirable for the good anode materials, which may be derived from micropore structure and not favorable for the transport of the solvent lithium ion. But in our case, the high specific BET surface area with large pore size could lead to sufficient electrode/electrolyte interface to absorb Li+ ions and promote rapid charge-transfer reaction, decrease the Li-ion diffusion length, enable additional electrochemical active sites and facilitate ultrafast surface lithium storage,23 resulting in favorable electrochemical performance of lithium ion batteries.
O, respectively.32,33 These C–O covalent bonds are result of the synergetic effect between the interface of SnO2 and carbon nanotubes, which not only perk up the structural firmness but also compose the hybrid structure more conductive as compared to the semiconducting SnO2.30 In Sn 3d spectrum (Fig. 2c), two symmetrical peaks at 486.9 eV and 495.4 eV are attributed to Sn 3d5/2 and Sn 3d3/2, respectively, which confirmed the formation of SnO2.34,35 To investigate the surface area and pore size distribution of the SnO2/CNT hybrids, Brunauer–Emmett–Teller (BET) method was carried out with a nitrogen adsorption–desorption process, as shown in Fig. 2d. The N2 adsorption–desorption isotherm demonstrates a typical type IV nitrogen adsorption branch with an H3 hysteresis loop, which indicates the porous structure,36,37 leading to a high BET surface area of 145.46 m2 g−1 with total pore volume of 0.6957 cm3 g−1. The pore size distribution was carried out by Barrett–Joyner–Halenda model, as depicted in the inset of Fig. 2d. The two peaks represent that the average pore sizes of SnO2 and CNT are measured to be 2.3 nm and 22.76 nm, respectively, which is consistent with the result of the TEM observation. It is proved by the experiments that a high BET surface area could be advantageous to the electrochemical performance of lithium ion batteries. Y. Dong et al. fabricated SnO2 nanoparticles fixed on graphene nanosheets with a high (BET) surface area of 295 m2 g−1, which exhibits good cyclic performance for lithium ion batteries.6 J. Chen and his coworkers prepared SnO2/Carbon composite having a high (BET) surface area of 375 m2 g−1, resulting in a high reversible average capacity used as anodes.44 Moreover, Y. P. Zhai et al. confirmed that the capacitance is in direct proportion to the electrode surface area according to a formula in their review.38 Compared with pure SnO2 nanosheets,23 SnO2/CNT hybrids have an increase in both surface area and pore size as expected. Generally speaking, the superhigh BET surface area value is not desirable for the good anode materials, which may be derived from micropore structure and not favorable for the transport of the solvent lithium ion. But in our case, the high specific BET surface area with large pore size could lead to sufficient electrode/electrolyte interface to absorb Li+ ions and promote rapid charge-transfer reaction, decrease the Li-ion diffusion length, enable additional electrochemical active sites and facilitate ultrafast surface lithium storage,23 resulting in favorable electrochemical performance of lithium ion batteries.
The electrochemical lithium storage properties of SnO2/CNT hybrids were evaluated as anode material for LIBs using lithium foil as reference electrode. The electrochemical reactivity were first tested by cyclic voltammetry (CV) measurement. Fig. 3a illustrates the CV curves of SnO2/CNT hybrids for the first five cycles at a scan rate of 0.1 mV s−1 with a potential range from 0–2.0 V (vs. Li+/Li). The electrochemical reactions of SnO2/CNT hybrids in LIBs were proposed as follows:15
| SnO2 + 4Li+ + 4e− → Sn + 2Li2O | (1) |
| Sn + xLi+ + xe− ↔ LixSn (0 ≤ x ≤ 4.4) | (2) |
| C (nanotube) + xLi+ + xe− ↔ LixC | (3) |
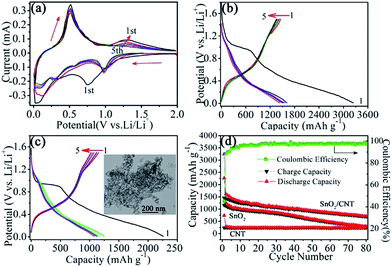
In the first cycle of cathodic process, two distinct reduction peaks appear at 0.75 and 0.02–0.45 V. The former one corresponds to the initial irreversible reduction of SnO2 to Sn and the synchronous formation of Li2O (eqn (1)), and solid electrolyte interphase (SEI) layer.23 The peaks between 0.02–0.45 V are ascribed to the creation of a series of LixSn alloys (eqn (2)) and lithium ions intercalating into the graphitic layers (eqn (3)).39 Compared with the pure SnO2 nanosheets, the reduction peaks locating at 0.02–0.45 V are much broader,23 indicating that the peculiar structure of SnO2/CNT hybrids could provide higher electrochemical activity for Li+ insertion.40 In the anodic process, the oxidation peaks at around 0.52 and 0.14 V stand for the reversible dealloying of LixSn (eqn (2)) and the lithium extraction from the CNTs (eqn (3)), respectively. It is worth noted that one obvious oxidation peak at 1.29 V shows up, indicating that eqn (1) is partially reversible, resulted from the decomposition of Li2O. Accordingly, the subsequent reduction peaks at 0.98 V are attributed to the formation of Li2O again.15 The CV curves become overlap after the first cycle, indicating a favourable reversibility of the electrochemical reaction of the electrode materials.
The electrochemical characteristics were additionally examined by charge–discharge tests with cycling at a current density of 200 mA g−1 within the potential range of 0.01–1.5 V (vs. Li+/Li) for 80 cycles. Fig. 3b and c show the representative discharge–charge voltage profiles of SnO2/CNT hybrids and pure SnO2 nanosheets, respectively. In both profiles, a long plateau emerged at about 0.95 V in the 1st discharge curves, which may be ascribed to the formation of SEI film and Li2O. The 1st discharge and charge capacities of the SnO2/CNT hybrids are 3247.5 and 1616.5 mA h g−1, respectively, which are much larger than the capacities of pure SnO2 nanosheets, 2260.7 and 1155.5 mA h g−1. The theoretical capacity for first cycle of such hybrid (85% SnO2 + 15% CNT) is 1053.8 mA h g−1, according to the reversible theoretical capacity of 782 mA h g−1 and the theoretical coulumbic efficiency for the first cycle of 52%.23 The theoretical capacity of SnO2 is 782 mA h g−1. Hu Y. and his co-workers have proved that the extra capacity is due to the constitution of a space charge layer comprised of lithium ions at the interface between lithium salt nanoparticles and the corresponding metal.41 The initial Coulombic efficiency of the hybrids is 49.8%, which is very close to the theoretical value 52%. The high initial lithium storage capacity could be ascribed to the distinct and unique structure of SnO2/CNT hybrids, where the contact between the SnO2 and the CNTs is quite tight, which can assemble a good quality conductive network to assist the electron transfer and provide more active sites and elastic buffer space for Li+ intercalation/de-intercalation, cut down the diffusion length for lithium ions, which helps in improving kinetic characteristics.42,43 By contrast, Chen and coworkers synthesized the similar material and obtained the initial discharge and charge capacity of 2038 and 1468 mA h g−1,44 whereas Mehmet et al. got initial discharge capacity of 1413 mA h g−1 for the similar material.39 L. Zhang et al. got initial discharge capacity of 2173 mA h g−1 for the similar hybrids.1 The discharge and charge capacities of SnO2/CNT hybrids in the 2nd cycle are 1658.4 and 1457.8 mA h g−1, respectively, resulting in an enhanced coulombic efficiency of 87.9% from the initial 49.8%. Moreover, the efficiency continued to increase up to 93.8% in the 5th cycle and kept on rising in the subsequent cycles. After the first discharge process, the SnO2/CNT hybrids show clearly overlapped discharge–charge curves, indicating an excellent cycling stability, which are consistent with the CV curves. By contrast, the pure SnO2 nanosheets exhibit separated curves, suggesting obviously capacity decay. The inset of Fig. 3c shows the TEM image of the pure SnO2 nanosheets for comparison.
Fig. 3d displays the comparative performances among SnO2/CNT hybrids, pure SnO2 nanosheets and carbon nanotubes. It is evident that SnO2/CNT hybrids marked a significantly enhanced lithium storage potential in comparison to the other two materials. After 80 charge and discharge cycles, a high reversible capacity of 710.4 mA h g−1 was demonstrated by SnO2/CNT hybrids, while the capacity of pure SnO2 nanosheets decreases seriously after 30 cycles and down to 288.8 mA h g−1 until the 80th cycle, almost approaching the reversible capacity of pure CNTs of 234.8 mA h g−1. In comparison, Jin and coworkers synthesized SnO2 nanocrystals incorporated with multiwalled carbon nanotubes using the hydrothermal method, the capacity falls to 420 mA h g−1 after 100 cycles at 156 mA g−1.31 Wu et al. synthesized the structure of SnO2/CNT nanocomposite anodes and the capacity was 462.5 mA h g−1 by 65 cycles at 100 mA g−1 current density.45
The greatly enhanced lithium storage capacity is mainly accredited to these reasons: (i) the arrangement of SnO2 nanosheets and nanoparticles around carbon nanotubes confines the mobility of the structure during cycling.42 (ii) the flexible CNTs improve the stability of SnO2 nanosheets and nanoparticles against agglomeration, which results in superior cyclability as compared to previous works on pure SnO2 nanosheets and nanorods.23,42 (iii) the unique construction of SnO2/CNT hybrids buffers the local volume gradient in Li–Sn alloying and de-alloying reactions well.20,46 (iv) Excellent electrical conductivity of CNTs is helpful to keep electrically connected with SnO2 during all charging and discharging processes; and (v) the large surface area and porosity of SnO2/CNT hybrids provided by both the interior and exterior of the hybrids are also beneficial to diffusion of lithium-ion.46 As a result, the use of a novel SnO2/CNT hybrids successfully addressed the fast capacity fading of pure SnO2 anodes and lead to a better electrochemical performance.
4. Conclusions
The novel SnO2/CNT hybrids have been effectively and successfully synthesized via an ultrafast and environmentally benign microwave-assisted method. The SnO2/CNT hybrids exhibit considerably enhanced reversible capacity in comparison with the bare SnO2 electrode due to the active function of the carbon nanotubes in the composites. Quite unlike the common approach of hydrothermal process requiring a high temperature and long time, the microwave-assisted method is timesaving, cost-effective, and can be accessed under atmospheric condition. The SnO2/CNT hybrids exhibited highly reversible capacity of 710.4 mA h g−1 up to 80 cycles, making it suitable as an active anode material for LIB. The improved electrochemical features can be associated with numerous unique features, including stress absorption by the carbon nanotubes, the increased electrical contact and enhanced lithium-ion transport.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21371023) and Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20101101110026).Notes and references
- L. Zhang, G. Zhang, H. B. Wu, L. Yu and X. W. Lou, Adv. Mater., 2013, 25, 2589 CrossRef CAS PubMed.
- K. Kang, G. Ceder, C. P. Grey, J. Bréger and Y. S. Meng, Science, 2006, 311, 977 CrossRef CAS PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- C. Guan, X. Wang, Q. Zhang, Z. Fan, H. Zhang and H. J. Fan, Nano Lett., 2014, 14, 4852 CrossRef CAS PubMed.
- J. Wang, N. Du, H. Zhang, J. Yu and D. Yang, J. Phys. Chem. C, 2011, 115, 11302 CAS.
- Y. Dong, Z. Zhao, Z. Wang, Y. Liu, X. Wang and J. Qiu, ACS Appl. Mater. Interfaces, 2015, 7, 2444 CAS.
- Y. Z. Su, S. Li, D. Q. Wu, F. Zhang, H. W. Liang, P. F. Gao, C. Cheng and X. L. Feng, ACS Nano, 2012, 6(9), 8349 CrossRef CAS PubMed.
- S. Li, D. Q. Wu, C. Cheng, J. Z. Wang, F. Zhang, Y. Z. Su and X. L. Feng, Angew. Chem., Int. Ed., 2013, 52, 12105 CrossRef CAS PubMed.
- S. Li, D. Q. Wu, H. W. Liang, J. Z. Wang, X. D. Zhuang, Y. Y. Mai, Y. Z. Su and X. L. Feng, ChemSusChem, 2014, 7(11), 3002 CrossRef CAS PubMed.
- Y. S. Huang, D. Q. Wu, S. Han, S. Li, L. Xiao, F. Zhang and X. L. Feng, ChemSusChem, 2013, 6, 1510 CrossRef CAS PubMed.
- Y. G. Zhu, J. Xie, G. S. Cao, T. J. Zhu and X. B. Zhao, RSC Adv., 2013, 3, 6787 RSC.
- M. Y. Wang, D. L. Jia, J. Li and J. G. Huang, RSC Adv., 2014, 4, 33981 RSC.
- J. C. Kim, I. S. Hwang, S. D. Seo, G. H. Lee, H. W. Shim, K. S. Park and D. W. Kim, Nanotechnology, 2012, 23, 789 Search PubMed.
- Y. Q. Zhu, C. Li and C. B. Cao, RSC Adv., 2013, 29, 11860 RSC.
- D. Wang, X. Li, J. Wang, J. Yang, D. Geng and R. Li, J. Phys. Chem. C, 2012, 116, 22149 CAS.
- S. Yang, W. Yue, J. Zhu, Y. Ren and X. Yang, Adv. Funct. Mater., 2013, 23, 3570 CrossRef CAS PubMed.
- G. Chen, Z. Wang and D. Xia, Chem. Mater., 2008, 20, 6951 CrossRef CAS.
- C. Xu, J. Sun and L. Gao, J. Phys. Chem. C, 2009, 113, 20509 CAS.
- S. Ding, D. Luan, F. Y. Boey, J. S. Chen and X. W. Lou, Chem. Commun., 2011, 25, 7155 RSC.
- X. W. Lou, C. M. Li and L. A. Archer, Adv. Mater., 2009, 21, 2536 CrossRef CAS PubMed.
- C. Wang, G. Du, K. Ståhl, H. Huang, Y. Zhong and Z. Jiang, J. Phys. Chem. C, 2012, 116, 4000 CAS.
- Z. Wen, Q. Wang, Q. Zhang and J. Li, Adv. Funct. Mater., 2007, 17, 2772 CrossRef CAS PubMed.
- Y. Q. Zhu and H. Guo, ACS Appl. Mater. Interfaces, 2015, 7, 2745 CAS.
- Y. Q. Zhu, H. Z. Guo, Y. Wu, C. B. Cao, S. Tao and Z. Wu, J. Mater. Chem. A, 2014, 2, 7904 CAS.
- Y. Q. Zhu, C. B. Cao, S. Tao, W. S. Chu, Z. Y. Wu and Y. D. Li, Sci. Rep., 2014, 4, 5787 CrossRef CAS PubMed.
- S. Khalid, C. B. Cao, A. Ahmad, L. Wang, M. Tanveer, I. Aslam, M. Tahir, F. Idrees and Y. Q. Zhu, RSC Adv., 2015, 5, 33146 RSC.
- Y. Q. Zhu, C. B. Cao, J. T. Zhang and X. Y. Xu, J. Mater. Chem. A, 2015, 3, 9556 CAS.
- J. A. Gerbec, D. Magana, A. Washington and G. F. Strouse, J. Am. Chem. Soc., 2005, 127, 15791 CrossRef CAS PubMed.
- M. O. Guler, O. Cevher, T. Cetinkaya, U. Tocoglu and H. Akbulut, Int. J. Energy Res., 2014, 38, 487 CrossRef CAS PubMed.
- L. Miao, J. B. Wu, J. J. Jiang and P. Liang, J. Phys. Chem. C, 2012, 117, 23 Search PubMed.
- Y. Jin, K. Min, S. Seo, H. Shim and D. Kim, J. Phys. Chem. C, 2011, 115, 22062 CAS.
- H. Huang, Y. Liu, Q. Gao, W. Ruan, X. Lin and X. Li, ACS Appl. Mater. Interfaces, 2014, 6, 10258 CAS.
- Y. M. Li, X. J. Lv, J. Lu and J. H. Li, J. Phys. Chem. C, 2010, 114, 21770 CAS.
- Y. Lin, X. Cui, C. Yen and C. M. Wai, J. Phys. Chem. B, 2005, 30, 109 Search PubMed.
- A. Velamakanni, C. W. Magnuson, J. Ganesh, K. Zhu, Y. An and P. J. Ferreira, ACS Nano, 2010, 4, 540 CrossRef CAS PubMed.
- L. Wang, Z. Y. Nie, C. B. Cao, M. W. Ji, L. Zhou and X. Feng, J. Mater. Chem. A, 2015, 3, 3710 CAS.
- L. Wang, Z. Y. Nie, C. B. Cao, Y. Q. Zhu and S. Khalid, J. Mater. Chem. A, 2015, 3, 6402 CAS.
- Y. P. Zhai, Y. Q. Dou, D. Y. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828 CrossRef CAS PubMed.
- O. G. Mehmet, O. Cevher, T. Cetinkaya, U. Tocoglu and H. Akbulut, Int. J. Energy Res., 2014, 38, 487 CrossRef PubMed.
- W. Ren, D. Kong and C. W. Cheng, ChemElectroChem, 2014, 1, 2064 CrossRef CAS PubMed.
- Y. Y. Hu, Z. Liu and K. W. Nam, Nat. Mater., 2013, 12, 1130 CrossRef CAS PubMed.
- S. M. Paek, Nano Lett., 2009, 9, 72 CrossRef CAS PubMed.
- P. Meduri, C. Pendyala, V. Kumar, G. U. Sumanasekera and M. K. Sunkara, Nano Lett., 2009, 9, 612 CrossRef CAS PubMed.
- J. Chen and K. Yano, ACS Appl. Mater. Interfaces, 2013, 5, 7682 CAS.
- P. Wu, N. Du, H. Zhang, J. Yu and D. Yang, J. Phys. Chem. C, 2010, 114, 22535 CAS.
- Y. Wang, H. Zeng and J. Lee, Adv. Mater., 2006, 18, 645 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |