Black TiO2 inverse opals for visible-light photocatalysis†
Linlin Xina and
Xuefeng Liu*b
aSchool of Chemical and Material Engineering, Jiangnan University, Wuxi, 214122, P. R. China
bThe Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, Jiangnan University, Wuxi, 214122, P. R. China. E-mail: xfliu@jiangnan.edu.cn; Fax: +86-510-85917763; Tel: +86-510-85917090
First published on 12th August 2015
Abstract
Black titanium dioxide (TiO2) inverse opals (BTIOs) were obtained by in situ H2 reduction of white TiO2 inverse opals (WTIOs). The combination of chemical hydrogenation and well-ordered inverse opal structure provides BTIOs with a better photocatalytic activity than the structure-related WTIOs, fragments of BTIOs, and P25 under visible light.
The expansion of the normal white TiO2 (WT) working spectrum to the visible-light region is currently of great interest.1 Recently, black TiO2 (BT), consisting of a crystalline stoichiometric TiO2 core and a disordered reduced self-doped shell2 or of a crystalline reduced TiO2 core (with oxygen vacancies) surrounded by a nearly stoichiometric amorphous shell3 has attracted plenty of attention due to its remarkable visible-light-driven photocatalytic activities.2–15 The improved visible-light photoresponse of BT is enabled by the self-doping Ti3+ defects2,3,6,15 and/or by external Ti–H and O–H bonds.4 In addition, the BT distorted outer shell creates an additional energy “tail” from the valence band into the band gap.2,3 Thus, BT opens new opportunities for visible-light active TiO2. However, inverse opals (IOs) of BT (BTIOs) have not been reported yet. IOs could provide WT with slow photon effect, a structural effect significantly different from the chemical composition effect.16–20 Thus, the combination of well-ordered inverse opal structure and chemical hydrogenation should enable BTIOs to enhance the light absorption and further improve the photocatalysis.
Herein, we report that BTIOs possess a better photocatalytic activity than the structure-related white TiO2 inverse opals (WTIOs), fragments of BTIOs (BT Fs), and P25 under visible-light (λ > 400 nm). BTIOs were obtained by reduction of WTIOs with H2 at 500 °C for 2 h. The corresponding WTIOs were fabricated by the forced impregnation method20 using monodisperse 360 ± 10 nm polystyrene (PS) nanosphere opal as the template and titanium isopropoxide as the Ti precursor.
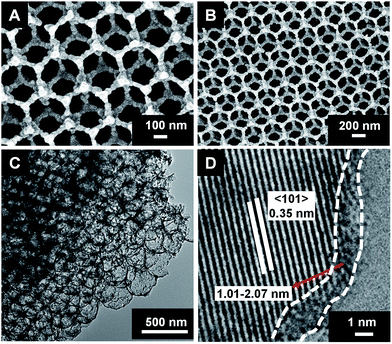
The field-emission scanning electron microscopy (FE-SEM) images (Fig. 1A and B) show that the PS opal template has a face-centered cubic (fcc) structure21 with a thickness of 10.4 μm (Fig. 1D). During the calcination at 500 °C under O2 atmosphere for 5 h, the PS template was removed completely, and highly ordered WTIOs was obtained (Fig. 1C and S1†).20 The spherical pores still exhibited an fcc structure, and the spherical pore size of WTIOs was approximately equal to 290 nm, revealing a shrinkage16,20 of approximately 19% compared with the diameter of the PS nanosphere template.
 | ||
| Fig. 1 FE-SEM image of PS opal (A and B) and the corresponding WTIOs (C and D) from top-view and side-view, respectively. | ||
After the hydrogenation, no significant changes were observed in the morphology, ordered structure, and average TiO2 grain size (approximately 35 ± 6 nm) of the BTIOs compared with those of the WTIOs (Fig. S2†).2–5,23 The transmission electron microscopy (TEM) image (Fig. 2C) illustrate the intact spatial periodicity of the multiple layers with close-packed spherical voids.20,22 The high-resolution TEM (HR-TEM) analysis (Fig. 2D) showed that the (101) crystal face spacing of BTIOs was ∼0.35 nm.7
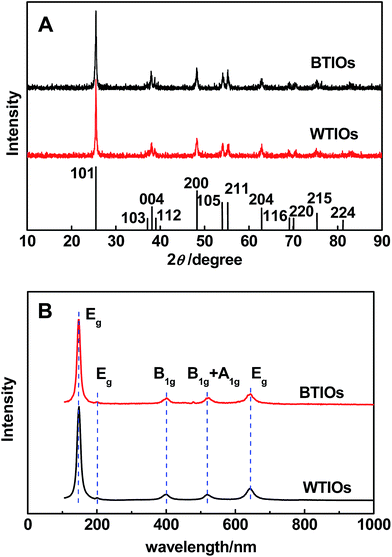
The X-ray diffraction (XRD) patterns (Fig. 3A) of BTIOs and WTIOs were indexed to typical anatase TiO2 (PDF#21-1272).17 Notably, no obvious changes between BTIOs and WTIOs were observed in the Raman spectra; all samples displayed typical anatase Raman bands with three Eg, two B1g, and one A1g active modes (Fig. 3B).2 All these results indicate that the hydrogenation of WTIOs does not modify the main crystal phase.6 Meanwhile, a disordered layer2–5 with a thickness of approximately 1.01–2.07 nm was observed on the surface of the BTIOs by HR-TEM (Fig. 2D).
The visible-light (λ > 400 nm) photocatalytic properties of BTIOs, WTIOs, and P25 were evaluated by the degradation of methylene blue (MB). To evaluate the effect of the inverse opal structure on the catalytic activity, the results for BTIOs fragments (BT Fs, Fig. S3†) are also shown in Fig. 4A. The apparent rate constants (k, ×102 h−1) of the catalytic reaction are 5.56 ± 0.33 (BTIOs), 4.55 ± 0.19 (BT Fs), 4.10 ± 0.18 (WTIOs), 2.81 ± 0.16 (P25), 1.35 ± 0.06 (BTIOs), respectively. The following order of BTIOs > BT Fs > WTIOs > P25 was observed. Ultraviolet-visible diffuse reflectance spectra (Fig. S4†) show that the band gaps of BTIOs and WTIOs are ∼2.77 eV5,9 and ∼3.08 eV,16 respectively. The value of the BTIOs band gap reported here is larger than that reported in the literature (<2 eV),3 which may be ascribed to a slight hydrogenation of the surface of our WTIOs; namely, the opals may comprise an anatase crystalline core and a very thin layer of a disordered shell (Fig. 2D). The specific surface areas (SBET, Fig. S5†) are 24.5 m2 g−1 for BTIOs, 11.7 m2 g−1 for WTIOs, and 49.5 m2 g−1 for P25, respectively. It is reported that the TiO2 unit cell contracts after hydrogenation.23 However, in this work, SBET of BTIOs is twice as large as that of the pristine WTIOs, which may be attributed to the presence of more active sites on the hydrogenated surfaces of BTIOs for N2 adsorption. Thus, BTIOs perform better than BT Fs, and WTIOs exhibit a superior behaviour to P25 (Fig. 4A), mainly because of the effect of the large-area well-ordered IO structure.16–19 Although the BT Fs has the same local structure of inverse opal as BTIOs (Fig. S3†), the long-range ordering of the well-ordered IO structure in BT Fs is partially destroyed. In contrast to the particles of micron-sized BT Fs and nano-sized P25, the stationary IO films of BTIOs and WTIOs have better mass transport under the stirring process of the MB degradation, owning to the higher rate of the shearing motion at the interface of the stationary IO film and the aqueous liquid of MB. BTIOs perform better than WTIOs, which is a reasonable result due to the surface hydrogenation;2,3,9 in addition, the superior photocatalytic properties of the BT Fs, compared to those of the WTIOs, indicate that the effect of the hydrogenation (chemical effect) on the visible-light photocatalytic activity of anatase TiO2 is possibly stronger than that of the well-ordered inverse opal structure (structural effect). Considering that SBET (P25) > SBET (WTIOs) and that P25 has a mixed crystal composition (∼80% of anatase and ∼20% of rutile), the SBET and doping of the narrower band gap crystal (for P25) may have a weaker impact on the visible-light photocatalytic properties of TiO2 than the abovementioned hydrogenation and well-ordered inverse opal effects. After recycling ten times, no obvious attenuation of the BTIOs photocatalytic activities was observed (Fig. 4B), and the value of relative standard deviation in MB degradation apparent rate constant was 5.96%, indicating that the structure (Fig. S6†) and the visible-light photoresponse of the BTIOs are stable. Although the BTIOs have not been optimized for greatest efficiency for the decolorization enhancement,15 they have shown that the combination of chemical hydrogenation and well-ordered inverse opal structure provides BTIOs with a better photocatalytic activity than the structure-related WTIOs, fragments of BTIOs, and P25 under visible light. A systematic investigation on the structural parameters of BTIOs (e.g., TiO2 grain size, spherical pore size and periodicity,18,19 and amorphous shell thickness), which can greatly affect the resulting visible-light driven photocatalysis enhancement, is under way.
 | ||
| Fig. 4 Degradation of methylene blue over BTIOs, WTIOs, BT Fs and P25 (A), and the apparent rate constant (k, h−1) of BTIOs in ten-time recycling (B). | ||
Conclusions
In summary, well-ordered BTIOs were obtained by in situ H2 reduction of the corresponding WTIOs, consisting of a crystalline TiO2 core with anatase phase and a disordered amorphous shell with a thickness of 1.01–2.07 nm. Our preliminary results show that the combination of chemical hydrogenation and well-ordered inverse opal structure provides BTIOs with a better photocatalytic activity than the structure-related WTIOs, fragments of BTIOs, and P25 under visible light. After recycling ten times, no obvious attenuation of the BTIOs photocatalytic activities was observed, indicating that the structure and the visible-light photoresponse of the BTIOs are stable.Acknowledgements
The authors acknowledge the financial support from National Science Foundation of China (grant no. 21071065), the Open Research Fund of the Key Laboratory of Food Colloids and Biotechnology Ministry of Education, Jiangnan University (JDSJ2012-07), and the Qinqlan Project of Jiangsu Province, China.Notes and references
- C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- X. B. Chen, L. Liu, P. Y. Yu and S. S. Mao, Science, 2011, 331, 746–750 CrossRef CAS PubMed.
- A. Naldoni, M. Allieta, S. Santangelo, M. Marelli, F. Fabbri, S. Cappelli, C. L. Bianchi, R. Psaro and V. Dal Santo, J. Am. Chem. Soc., 2012, 134, 7600–7603 CrossRef CAS PubMed.
- Z. Zheng, B. Huang, J. Lu, Z. Wang, X. Qin, X. Zhang, Y. Dai and M.-H. Whangbo, Chem. Commun., 2012, 48, 5733–5735 RSC.
- F. Teng, M. Li, C. Gao, G. Zhang, P. Zhang, Y. Wang, L. Chen and E. Xie, Appl. Catal., B, 2014, 148–149, 339–343 CrossRef CAS PubMed.
- F. Zuo, L. Wang, T. Wu, Z. Zhang, D. Borchardt and P. Feng, J. Am. Chem. Soc., 2010, 132, 11856–11857 CrossRef CAS PubMed.
- J. Dong, J. Han, Y. Liu, A. Nakajima, S. Matsushita, S. Wei and W. Gao, ACS Appl. Mater. Interfaces, 2014, 6, 1385–1388 CAS.
- Z. Pei, L. Ding, W. Feng, S. Weng and P. Liu, Phys. Chem. Chem. Phys., 2014, 16, 21876–21881 RSC.
- Z. Wang, C. Yang, T. Lin, H. Yin, P. Chen, D. Wan, F. Xu, F. Huang, J. Lin, X. Xie and M. Jiang, Adv. Funct. Mater., 2013, 23, 5444–5450 CrossRef CAS PubMed.
- T. Leshuk, R. Parviz, P. Everett, H. Krishnakumar, R. A. Varin and F. Gu, ACS Appl. Mater. Interfaces, 2013, 5, 1892–1895 CAS.
- Y. Yan, M. Han, A. Konkin, T. Koppe, D. Wang, T. Andreu, G. Chen, U. Vetter, J. R. Morantee and P. Schaaf, J. Mater. Chem. A, 2014, 2, 12708–12716 CAS.
- H. Cui, W. Zhao, C. Yang, H. Yin, T. Lin, Y. Shan, Y. Xie, H. Gu and F. Huang, J. Mater. Chem. A, 2014, 2, 8612–8616 CAS.
- N. Liu, C. Schneider, D. Freitag, M. Hartmann, U. Venkatesan, J. Müller, E. Spiecker and P. Schmuki, Nano Lett., 2014, 14, 3309–3313 CrossRef CAS PubMed.
- W. Zhou, W. Li, J.-Q. Wang, Y. Qu, Y. Yang, Y. Xie, K. Zhang, L. Wang, H. Fu and D. Zhao, J. Am. Chem. Soc., 2014, 136, 9280–9283 CrossRef CAS PubMed.
- B. Chen, J. A. Beach, D. Maurya, R. B. Moore and S. Priya, RSC Adv., 2014, 4, 29443–29449 RSC.
- M. Wu, J. Liu, J. Jin, C. Wang, S. Huang, Z. Deng, Y. Li and B.-L. Su, Appl. Catal., B, 2014, 150–151, 411–420 CrossRef CAS PubMed.
- M. Wu, J. Jin, J. Liu, Z. Deng, Y. Li, O. Deparis and B.-L. Su, J. Mater. Chem. A, 2013, 1, 15491–15500 CAS.
- J. I. L. Chen, G. von Freymann, S. Y. Choi, V. Kitaev and G. A. Ozin, Adv. Mater., 2006, 18, 1915–1919 CrossRef CAS PubMed.
- J. I. L. Chen and G. A. Ozin, J. Mater. Chem., 2009, 19, 2675–2678 RSC.
- X. Chen, Z. Li, J. Ye and Z. Zou, Chem. Mater., 2010, 22, 3583–3585 CrossRef CAS.
- P. Jiang, J. F. Bertone, K. S. Hwang and V. L. Colvin, Chem. Mater., 1999, 11, 2132–2140 CrossRef CAS.
- W. Li, Z. Wu, J. Wang, A. A. Elzatahry and D. Zhao, Chem. Mater., 2014, 26, 287–298 CrossRef CAS.
- T. Xia and X. Chen, J. Mater. Chem. A, 2013, 1, 2983–2989 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and additional characterization figures and data, including UV-vis and BET analysis. See DOI: 10.1039/c5ra10280d |
| This journal is © The Royal Society of Chemistry 2015 |