A novel phenylacetylene-indolium fluorophore for detection of cyanide by the naked eye†
Abstract
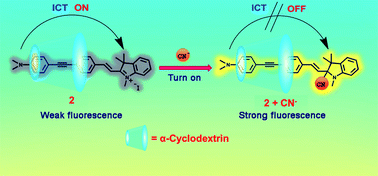
A novel fluorescent turn-on cyanide sensor containing an indolium salt as the selective CN− receptor was synthesized in 2 steps via Sonogashira coupling and Knoevenagel reaction. Compound 2 was developed as a new fluorometric and colorimetric sensor for cyanide with a sensing mechanism through cyanide ion addition to the indolium group. Importantly, the inclusion complexes between compound 2 and α-cyclodrextin, exhibited fluorescent turn-on detection of CN− in 100% aqueous media with the detection limit of 1.3 μM. The turn-on signal is the result of the conversion of the conjugated hemicyanine moiety to a cyano-hemicyanine group via the addition of the cyanide ion. Using paper sensor strips, naked eye detection of cyanide is possible down to 4.59 ng mm−2. Test strips based on compound 2 can act as a convenient and efficient CN− testing kit.

 Please wait while we load your content...
Please wait while we load your content...