The efficient adsorption removal of Cr(vi) by using Fe3O4 nanoparticles hybridized with carbonaceous materials
Abstract
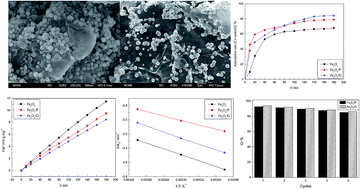
Fe3O4 nanoparticles hybridized with carbonaceous materials, such as pinecone and graphene, were successfully synthesized by a facile hydrothermal method, which could be applied for Cr(VI) removal in aqueous solution. The nanocomposites were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and N2 adsorption–desorption isotherms. Due to the combination of pinecone and graphene, both the surface properties and morphologies of Fe3O4 were modified. Fe3O4 spherical particles were distributed and firmly anchored on the loose surface of pinecone or wrinkled graphene layers. The specific surface area increased from 23.85 to 27.86 and 121.17 m2 g−1 for Fe3O4/P and Fe3O4/G, respectively. It enhanced the adsorption capacity for Cr(VI) of Fe3O4/P (62.5 mg g−1) and Fe3O4/G (78.5 mg g−1). Study of the kinetics and isotherms showed that the pseudo-second-order kinetic and Langmuir isotherm models fitted the adsorption data well. There were three steps in the adsorption process, namely an instantaneous adsorption step, intraparticle diffusion and a final equilibrium stage. The reaction rate decreased along with temperature increasing, which indicated that Cr(VI) adsorption was an exothermic process. The Ea values were 34.39, 25.77 and 34.92 kJ mol−1 for Fe3O4, Fe3O4/P and Fe3O4/G, respectively, which illustrated that the adsorption of Cr(VI) onto the surface of the nanocomposites was a physical process. In no more than 5 h, about 92.6% and 94% Cr(VI) were desorbed from the surface of Fe3O4/P and Fe3O4/G, respectively, which indicated that the adsorption–desorption process for Cr(VI) was reversible. The results demonstrated that Fe3O4/P and Fe3O4/G exhibited excellent adsorption performance in the removal of Cr(VI). It was proved that carbonaceous materials, such as pinecone or graphene, could enhance the adsorption performance of Fe3O4, and could be used as adsorbents to remove heavy metals in industrial effluents.

 Please wait while we load your content...
Please wait while we load your content...