From laboratory catalysts to a new prototype: a novel real candidate for the isomerization of C5–C6 paraffins†
J. M. Hidalgo*a,
D. Kauckýc,
O. Bortnovskyb,
R. Černýa and
Z. Sobalíkc
aResearch Institute of Inorganic Chemistry - VUANCH, Research and Development, Czech Republic. E-mail: jose.hidalgo@vuanch.cz; Tel: +420 731 534 979
bEurosupport Manufacturing Czechia, Czech Republic
cJ. Heyrovsky Institute of Physical Chemistry, Catalysis, Czech Republic
First published on 16th June 2015
Abstract
Many authors have published much information about the isomerization of paraffins C5–C6 using Pt/WO3–ZrO2 materials. Nevertheless, only model feedstocks have been used. An active catalyst was developed with the aim of isomerising industrial feedstocks. This material presented stable activity during the reaction with an increase of the R.O.N. of 14 at 225 °C. The material is the first real candidate for a tungstated zirconia to be used in industry to isomerise light paraffins.
Since WO3–ZrO2 materials were discovered by Hino and Arata,1,2 a large amount of research work about these materials has been carried out. The structure, strong acidity and WOx species1–10 were found to be important for their activity in the isomerization reaction of light paraffins and their platinum content to activate-dissociate the molecular hydrogen and/or hydrogenate olefinic intermediates.9–11 The hydro-isomerization of paraffins is an important reaction for the production of quality gasoline and diesel fuels.5–8 Zirconia catalysts (Pt/WOx–ZrO2) were intended to remove disadvantages related to environmental concerns of traditional commercial alumina-based7,8 catalysts doped with chlorine and at the same time to reach sufficient activity and selectivity for n-alkane skeletal isomerization.11–14 Nevertheless, the published works were carried out using short reaction times and model feeds or simulated reformate feedstock.11 A publication from our research group presented results comparing the use of model and real industrial feedstock15 obtaining a lower increase of the research octane number when the real feedstock was used. Additionally, many articles published were carried out using different conditions than those used by industry, and most of these experiments were carried out in the laboratory using small reactors and only a few milligrams of the catalysts.16–22 So, the extrapolation of these lab-results to industry might not be the most appropriate way. However, all the previous studies were crucial and necessary to continue with other experiments closer to industry. Thanks to all these previous publications, the production of a new prototype was possible.
Several results were generated and published by our research-team during the development of the new prototype for the isomerization of C5–C6 paraffins.15,23,24 These results could be taken as an example of the development process of the prototype. The syntheses of catalysts previous to the prototype and their reaction conditions were carried out according to the published literature15,17,23,24, taking into account the conditions used by industry.25
From previous information11,26 about the positive effect of the addition of Al to the tungstated zirconia catalysts and our previous experience,24 several catalysts were synthesized. Nevertheless, the process of synthesis was different than that used in literature.11,26 In this case, the aluminum was not included in the framework of the zirconia oxide11 or in the WO3–ZrO2 structure.26 The solids were prepared by impregnation of hydrous zirconium oxide-hydroxide ZrO(OH)2, provided by the company Euro Support Manufacturing Czechia, using a previously described method by D. Kaucký et al.17 The hydrous zirconium oxide-hydroxide aqueous solution was dried at 423 or 573 K for 2 h. Then, aluminum nitrate and (NH4)6H2W12O40 were added to the solid and the mixture was dried at 423 or 573 K for 2 h. Then, the solid was calcinated at 973 K for 1–3 h. Finally, platinum(II) nitrate was impregnated over the solid. Different amounts of the components of the synthesis were added to obtain the content of 24 wt% of WO3, 1.5 or 3 wt% of Al2O3 and 0.25 wt% of Pt. Finally, all the catalysts were pelletized.
The syntheses were carried out in the laboratory and a pilot plant by the company Euro Support Manufacturing Czechia. The procedure was the same but using larger amounts of raw materials and using bigger mechanical equipment such as the kneading machine of a lab-mixer.
Eight catalysts were synthesized, from a first sample synthesized in the laboratory (Pt-Lab) to a prototype synthesized in a pilot plant in the company Euro Support Manufacturing Czechia (Table 1). Several changes in the synthesis, binder and temperature of drying (in the different steps of drying described above) were carried out with the aim of studying their influence on the final activity of the catalyst.
| Name of the catalyst | Temperature of drying (K) | Binder | Amount of Al2O3 |
|---|---|---|---|
| a The prototype was synthesized in the same way as the Pt-Pilot-G catalyst but with a time of calcination of 3 h at 973 K.b “Pilot” means that the catalyst was synthesized in a pilot plant in the company Euro Support Manufacturing Czechia. | |||
| Pt-Lab | 423 | — | 1.5 |
| Pt-Lab-2 | 573 | — | 1.5 |
| Pt-Lab-3 | 573 | — | 3 |
| Pt-Pilotb | 423 | — | 1.5 |
| Pt-Pilot-G | 423 | Graphite | 1.5 |
| Pt-Pilot-S | 423 | Al–stearate | 1.5 |
| Prototypea | 423 | Graphite | 1.5 |
The catalysts were characterized through mercury porosimetry intrusion using a mercury porosimeter, Micromeritics AutoPore IV 9500. Diffuse-reflectance UV-Vis spectra of samples were recorded on a Perkin-Elmer Lambda 950 spectrometer. Raman spectra (0–3000 cm−1) were measured at room temperature using a Thermo Scientific DXR Raman Microscope Class 1 Laser Product. HR-TEM images were obtained on a HRTEM JEOL JEM 3010 microscope.
A trickle bed reactor with a length of 1000 mm and inner diameter of 17 mm was used for the test. The catalysts prepared in the form of pellets with sizes 1.2–1.5 mm were crushed into pieces of 0.25–0.5 mm for the catalytic test. The catalyst bed had a length of 301 mm. A small amount of glass wool was placed at the bottom of the reactor, and covered by 10 mL of pure silicon carbide. The mixtures of the catalyst with silicon carbide (0.5 mm particles) (20 mL SiC + 20 mL catalyst) were mixed thoroughly and loaded into the reactor. A hydrogen stream, used for catalyst reduction and during the catalytic reaction, was fed directly or mixed with feedstock before entering the reactor. Catalysts were activated. At the beginning the catalyst was heated from room temperature to 748 K (5 K min−1) (air flow 30 NL h−1), followed by a time lag of 3 hours at 748 K. Then, the reactor was cooled to 523 K using a nitrogen flow (50 NL h−1). Finally, the catalyst was reduced in an H2 stream at 523 K for 2 hours. Catalytic tests were carried out at 523 K and a pressure of 15 bar (H2), using 30 g of n-alkane per hour fed by a HPLC pump (feedstock), with 30.4 NL h−1 of H2. The components used for the feeds were: n-pentane (98% wt); n-hexane (99% wt); a mixture of 45 wt% of n-hexane, 35 wt% of C6 isomers, 16 wt% of methylcyclopentane and 2 wt% of others (denoted as hexane pure); and an industrial feedstock, i.e. a complex mixture rich in nC5–nC6 but containing also iC5–iC6 paraffins, and other components.
Reaction products were sampled for on-line GC analysis (gas chromatograph Shimadzu GC17A equipped with a HP-Pona Column (50 m × 0.25 mm) with nitrogen carrier gas), and collected for further analysis in the first collector at room temperature (cooled by water) and in the second collector (at 248 K) (Fig. 2). The collected liquids were analyzed separately by gas chromatography. The GC Agilent 7890A and the software DHA (Detailed Hydrocarbon Analysis) were used to quantify and identify all the compounds present in the raw material and the reaction products, and to calculate the Research Octane Number – R.O.N.
The total surface area was only 64 m2 g−1 for the final prototype catalyst. Nevertheless the value for the surface area between 10 and 50 nm was the second highest for the final prototype. So, the catalyst with the highest activity (prototype) presented a high surface area in this area (Table 2).
| Catalyst name | Total surface area | Surface areaa (3–50) | Surface areaa (3–10) | Surface area (10–50) |
|---|---|---|---|---|
| a Calculated surface area of pores with size 3–50, 3–10 or 10–50 nm respectively. | ||||
| Pt-Lab | 73 | 63 | 22 | 41 |
| Pt-Lab-2 | 71 | 66 | 35 | 31 |
| Pt-Lab-3 | 68 | 63 | 33 | 30 |
| Pt-Pilot | 57 | 48 | 14 | 34 |
| Pt-Pilot-G | 84 | 77 | 29 | 48 |
| Pt-Pilot-S | 90 | 87 | 42 | 45 |
| Prototype | 64 | 64 | 17 | 46 |
The types of WOx species were mainly mononuclear ones in all cases. The catalysts prepared in the pilot plant presented the same amount of polynuclear species.
The Raman spectra complemented the results obtained by UV-vis (Table 3), and they could be considered only as qualitative analyses. The signals were assigned according to the data published by Vaudagna et al.13 The bands at 170 cm−1 were assigned to the ZrO2, at 480 and 616 cm−1 to monoclinic and tetragonal structures of ZrO2, respectively. The signals at 715 cm−1 and 807 cm−1 were assigned to the bending and stretching vibrations of W–O, respectively. The signal at 980 cm−1 could be interpreted as representing the WO3–Al2O3 or assigned to the stretching of W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species.
O species.
| Catalyst name | Types of WOx species (relative%) | ||
|---|---|---|---|
| Mononuclear | Polynuclear | Bulk-like | |
| a The amount of binders included in the Pt-Pilot catalysts was 2 wt%. They were mixed directly with the synthesized catalyst. | |||
| Pt-Lab | 41.8 | 42.8 | 15.4 |
| Pt-Lab-2 | 57.8 | 27.5 | 14.7 |
| Pt-Lab-3 | 58.0 | 29.5 | 12.4 |
| Pt-Pilot | 58.5 | 26.8 | 14.7 |
| Pt-Pilot-G | 55.0 | 30.0 | 15.0 |
| Pt-Pilot-S | 60.0 | 30.0 | 10.0 |
| Prototype | 50.0 | 30.0 | 20.0 |
The HR-TEM showed images from 50 to 5 nm but the detection of Pt particles or W particles was not clear (Fig. 1).
The results obtained in the isomerization of n-hexane (Table 4) showed a high increase of the R.O.N. for the prototype and for the Pt-Pilot-G catalysts. The lowest activities were found for the catalyst Pt-Lab-2 and Pt-Pilot-S. The addition of aluminum stearate caused a decrease in the activity of the catalyst (Pt-Pilot-G) and the drying at 573 K resulted in lower yields of iso-hexanes. The increase of the amount of aluminium from 1.5 to 3 wt% of Al2O3 (from Pt-Lab-2 to Pt-Lab-3) resulted in an increase of the activity. When a new sample which contained more aluminium than the sample Pt-Lab was created, an increase of the activity could be expected but the increase resulted (results not shown in this manuscript) in a dramatic decrease in the activity. The introduction of the binder graphite improved the activity of the catalyst and also improved the mechanical properties of the pellets (without binders the pellets were too fragile and crushable).
| Catalyst | TOS (h)/Xh (wt%)/R.O.N increase | TOS (h)/Xh (wt%)/R.O.N increase |
|---|---|---|
| Pt-Lab | 72/43/13 | 96/43/13 |
| Pt-Lab-2 | 49/16/3 | 70/14/3 |
| Pt-Lab-3 | 97/30/10 | 115/30/10 |
| Pt-Pilot | 73/38/12 | 91/38/12 |
| Pt-Pilot-G | 72/47/17 | 96/45/17 |
| Pt-Pilot-S | 34/18.8/4.5 | 43/17.7/4 |
| Prototype | 72/57/18 | 96/57/18 |
The use of aluminium stearate as binder caused a decrease in the activity of isomerization from nC6 to iC6. In all cases, the yield of the hydrocracking reaction (using hexane pure or C5–C6 feeds) at 498 K to C1–C5 products was lower than 1 wt%.
The prototype presented the maximum activity compared to the other catalysts, using hexane pure and C5–C6 feedstock, with an increase of the R.O.N. of 20 (hexane pure; TOS = 24 h) and 17 (C5–C6 feedstock; TOS = 48 h). Nevertheless, the activity using hexane pure or C5–C6 feed, decreased to an increase of the R.O.N. of 18 or 15 (with a slight decrease of the activity from 15 to 13 from 144 to 480 h as shown in Fig. 2) respectively. The catalyst was tested for 480 h continuously.
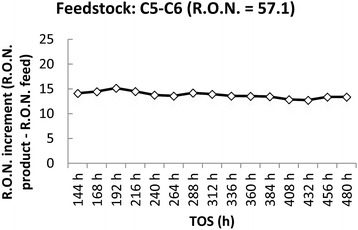 | ||
| Fig. 2 R.O.N. increase from feedstock to product using C5–C6 feedstock. T = 498 K; feed flow = 30 g h−1; catalyst amount = 20 g; hydrogen gas flow = 30 NL h−1; pressure = 15 bar. | ||
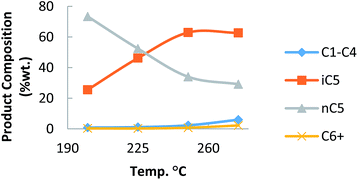
To know exactly the isomerization activity using n-pentane (Fig. 3), the catalyst was tested at different temperatures.
The hydrocracking reaction occurred minimally, but increased from 250 °C. The capacity of production of isomers iC5 from nC5 was found to be between that of commercial sulfated zirconia and zeolites (Fig. 4).25 The present results demonstrated the potential of this catalyst as possible alternative to traditional catalysts.
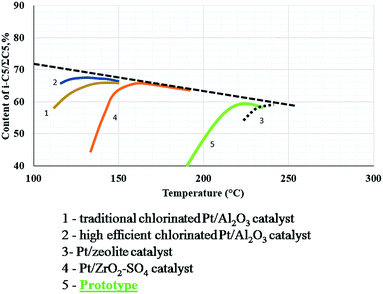 | ||
| Fig. 4 Comparative performance of isomerization catalysts.25 The prototype sample was compared to commercial catalysts (figure adapted from E. A. Yasakova and A. V. Sitdikova25). | ||
The conclusions are that different catalysts based on tungstated zirconia were synthesized according to the information from literature and previous experience of the team’s work. The best material was selected as a prototype, and is a real alternative to traditional commercial catalysts. Its activity could be situated between that of commercial sulfated zirconia and the zeolite catalysts.
Acknowledgements
This publication is a result of the project no. FR-TI3/316 which is being carried out with the support of Ministry of Industry and Trade of the Czech Republic in the UniCRE centre, which infrastructure was supported by the European Regional Development Fund and the state budget of the Czech Republic.Notes and references
- M. Hino and K. Arata, Chem. Commun., 1988, 1259 RSC.
- M. Hino and K. Arata, Appl. Catal., A, 1998, 169, 151 CrossRef CAS.
- D. G. Barton, S. L. Soled, G. D. Meitzner, G. A. Fuentes and E. Iglesia, J. Catal., 1999, 181, 57 CrossRef CAS.
- I. E. Wachs, Catal. Today, 1996, 27, 437 CrossRef CAS.
- S. Kuba, P. Lukinskas, R. K. Grasselli, B. C. Gates and H. Knözinger, J. Catal., 2003, 216, 353 CrossRef CAS.
- S. Kuba, P. Lukinskas, R. Ahmad, F. C. Jentoft, R. K. Gates and H. Knözinger, J. Catal., 2003, 219, 376 CrossRef CAS.
- H. Hattori, T. Yamada and S. Triwahyono, Participation of the Protonic Acid Sites Originating from Molecular Hydrogen in Alkane Skeletal Isomerization Catalyzed by Pt/WO3–ZrO2. Proceed. 3rd Saudi-Japanese Cat. Symp., Sapporo 060–8628, Japan, Dhahran, 2003.
- J. M. Hidalgo, M. Zbuzek, R. Černý and P. Jíša, Cent. Eur. J. Chem., 2014, 12, 1 CrossRef CAS PubMed.
- K. S. Anisia, G. S. Mishra and A. Kumar, J. Mol. Catal. A: Chem., 2004, 215, 121 CrossRef CAS PubMed.
- D. Maxa, VŠCHT Praha, Ústav technologie ropy a alternativních paliv, 2011, vol. 2011, http://www.petroleum.cz.
- J. Xu and J. Y. Ying, Angew. Chem., Int. Ed., 2006, 45, 6700 CrossRef PubMed.
- S. Cavanese, Z. Finelli, M. Busto, V. M. Benitez, C. R. Vera and J. C. Yori, Quim. Nova, 2010, 33, 508 CrossRef PubMed.
- S. R. Vaudagna, R. A. Comelli and N. S. Fígoli, Appl. Catal., A, 1997, 164, 265 CrossRef CAS.
- M. G. Falco, J. M. Grau and N. S. Figoli, Appl. Catal., A, 2004, 264, 183 CrossRef CAS PubMed.
- J. M. Hidalgo, D. Kaucky, O. Bortnovsky, R. Černy and Z. Sobalik, Monatsh. Chem., 2014, 145, 1407 CrossRef CAS PubMed.
- K. Song, Z. Hongbin, Y. Zhang, Y. Tang and K. Tang, J. Catal., 2013, 299, 119 CrossRef CAS PubMed.
- D. Kaucký, B. Wichterlová, J. Dedecek, Z. Sobalík and I. Jakubec, Appl. Catal., A, 2011, 397, 82 CrossRef PubMed.
- M. M. Antunes, S. Lima, A. Fernandes, J. Candeias, M. Pillinger, S. M. Rocha, M. F. Ribeiro and A. A. Valente, Catal. Today, 2012, 195, 127 CrossRef CAS PubMed.
- N. Soultanidis, W. Zhou, A. C. Psarras, A. J. Gonzalez, E. F. Iliopoulou, C. J. Kiely, I. E. Wachs and M. S. Wong, J. Am. Chem. Soc., 2010, 132, 13462 CrossRef CAS PubMed.
- M. Busto, L. A. Dosso, C. R. Vera and J. M. Grau, Fuel Process. Technol., 2012, 104, 128 CrossRef CAS PubMed.
- G. X. Yu, D. L. Lin, Y. Hu, X. Zhou, C. L. Li, L. F. Chen and J. Wang, Catal. Today, 2011, 166, 84 CrossRef CAS PubMed.
- M. L. Hernández-Pichardo, J. A. Montoya, P. Angel, A. Vargas and J. Navarrete, Appl. Catal., A, 2008, 345, 233 CrossRef PubMed.
- R. Černý, J. M. Hidalgo, O. Bortnovsky and D. Kaucky, Moscow, Russia, 7–12 July 2013, 17th International Zeolite Conference, P-3.1-33, Experimental evaluation of hexane isomerization over Pt/WOx–ZrO2 catalysts.
- J. M. Hidalgo, D. Kaucky, O. Bortnovsky, R. Cerny, Z. Sobalik and P. Sazama, 1–6 Jun 2014, the Seventh Tokyo Conference on Advanced Catalytic Science and Technology (TOCAT7), Tailoring of The Structure of Pt/WO3-ZrO2 Catalyst for High Activity in Skeletal Isomerisation of C5-C6 Paraffins under Industrially Relevant Conditions.
- E. A. Yasakova and A. V. Sitdikova, Oil and Gas Business, 2010, UDC 665.656.2 Search PubMed.
- X. Chen, Y. Du, C. Chen, N. Xu and C. Mou, Catal. Lett., 2006, 111, 3 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra10101h |
| This journal is © The Royal Society of Chemistry 2015 |