Energy transfer properties of a novel boron dipyrromethene–perylenediimide donor–acceptor dyad†
Abstract
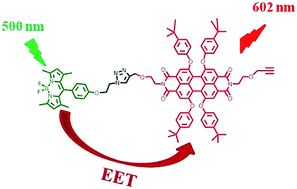
Borron dipyrromethenes (BDPs) and perylenediimides (PDIs) are excellent building blocks for the design of artificial light-harvesting systems. In the present work, we report the results of photophysical studies of a novel dyad, in which BDP and PDI chromophores are covalently linked to each other via a 4-(ethoxymethyl)-1-(phenoxyethyl)-1,2,3-triazole unit. It was found that efficient excitation energy transfer (EET) from the initially photoexcited BDP moiety to the PDI chromophore in its ground state resulted in strong quenching of the BDP first excited singlet state as well as in the appearance of the PDI fluorescence. The efficiency of EET was calculated to be 0.99 and the rate of this process was found to be 1.85 × 1010 s−1.

 Please wait while we load your content...
Please wait while we load your content...