DOI:
10.1039/C5RA10062C
(Review Article)
RSC Adv., 2015,
5, 79129-79151
Recent advances in hybrid periodic mesostructured organosilica materials: opportunities from fundamental to biomedical applications
Received
28th May 2015
, Accepted 10th September 2015
First published on 11th September 2015
Abstract
Surfactant-mediated periodic mesoporous organosilicas (PMOs) with different organic and inorganic functions in the framework structure were discovered in 1999. The silica inorganic function gives a strong mechanical stability to the framework structure and the organic functionality creates more structural flexibility. As a result, their use is optimal for a large number of applications including catalysis, microelectronics, chromatographic supports, selective adsorbents, sensors, protein folding, biomedical devices and light-harvesting devices. These new materials showed also superior stability when compared to pure mesoporous silica (PMS) framework structures. Based on the recent discoveries, since 2012, this review mostly provides a comprehensive overview of their multidisciplinary options for various applications.
 Mohammad A. Wahab | Wahab has received his BSc (Hons) and MSc in Chemistry from University of Rajshahi, Bangladesh. He has earned his PhD degree in Materials Chemistry and Polymer Engineering from Pusan National University in South Korea. His PhD study focuses on the design, self-assembly and application of periodic mesoporous organosilica nanostructures and nanohybrids. Then Dr Wahab worked as Research Scientist in IMRE-A*STAR (Agency for Science Technology and Research) Singapore with Prof. Alan Sellinger and Prof. He Chaobin for 5 years. In 2010, he moved to ARC Centre for Functional Nanomaterials, AIBN, The University of Queensland in 2010. His research interests mainly focus on design, synthesis and application of various nanostructured porous materials including PMO, carbon, carbon nitride and metal functionalized carbon nitride for catalysis, energy storage, immobilization and biomedical and electrochemical applications. |
 Jorge N. Beltramini | Dr Jorge Beltramini is a Senior Research Fellow at the ARC Centre for Functional Nanomaterials at the University of Queensland, Australia with more than 30 years of experience in catalysis, surface characterization and reactor design. He has co-authored more than 150 journal and international conference papers. His present research interests include the synthesis, manipulation and catalytic characterization of new nanomaterials for natural gas, coal, and biomass conversion into fuels, valuable chemicals and clean hydrogen production. |
1. Introduction
Mesoporous materials synthesized via self-assembly techniques are widely used in advanced applications such as: optoelectronics, light harvesting, biomedical applications, sensors, catalysts, environmental applications, and nanobiotechnology.1–24 Their use depends on the pore size and shape and on the presence of functional groups in their final mesoporous framework.5–22 In 1992, the discovery of pure mesoporous silica structures (known as MCM41, where MCM stands Mobil Composition of Matter) via the surfactant-mediated self-assembly synthesis of surfactant micelles and tetraethyl orthosilicate (TEOS) expanded the applications of microporous molecular sieves beyond the 1–2 nm restrictions.1 At the same time, by changing the surfactant concentration and synthesis temperatures a new kind of ordered mesoporous silica materials including MCM48 (cubic pore) and MCM50 (lamellar/layered) were also reported.1 The removal of the surfactant micelles by calcination or solvent extraction leaves behind well-ordered porous mesostructured silica (PMS) structures with pores diameters ranging from 2 to 5 nm and specific surface areas of about 900–1000 m2 g−1. In 1998, silica based SBA15 and associated similar kind of structures with different wall thicknesses were also reported.11–16,16–24 Efforts were also made to overcome the neutral nature of inorganic mesoporous silica framework by functionalization with various functional groups to expand their applications in different research areas.1–34 Another important limitation that concerns with the hydro-thermal stability of PMS structures is when they are present in a water/aqueous medium. For example, if MCM41/MCM48 samples are exposed to boiling water for 12 h then they will completely loss their framework structure due to the hydrolysis of the silicate walls (Si–O–Si). Hence, these mesoporous silica structural limitations made necessary the design and development of new alternative materials that can overcome the aforementioned drawbacks.1–5,15–30 In this context, hybrid PMOs prepared by self-assembling organic and inorganic units (Fig. 1) in a single material framework can easily overcome the limitations found on PMS materials.5,15–23,40–84 Significantly, the presence of inorganic Si function in the framework structure usually builds a robust framework while organic functions make frameworks more flexible in a way so that PMOs can be possible tailored in a number of important applications.20–105 For the comfort of the reader, a list of acronyms used in this review is outlined at the end of text.
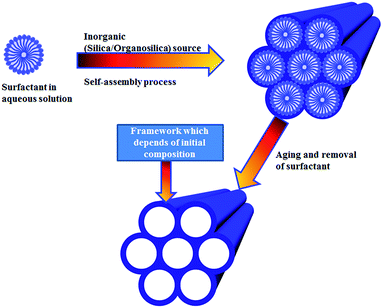 |
| | Fig. 1 General self-assembly method for preparing PMOs. Framework composition of the final PMOs depends on the initial reactant components. | |
2. Limitations of pure mesoporous silicas lead to discover PMOs
2.1 Functionalization of mesoporous silica framework
Two synthesis techniques were used that can explain the functionality of various PMOs. Firstly, co-condensation method (Fig. 2) which is associated with the co-assembling of TEOS with an organosiloxane of the type RSi(OEt)3 (R stands for organic group and OEt stands for ethoxy group) in the presence of surfactant under acid or basic conditions, and, secondly post-grafting technique (Fig. 3) where the organic functional groups are usually incorporated onto the pore channel walls of mesoporous framework taking advantage of the reactivity of the surface silanol groups (Si-OH) present into the mesoporous structure.15–18,20–26 As a result the organic groups became a part of the wall structure, providing functionality not only to its surface but to the entire mesoporous structure.6–14,49–79 However, both methods suffer from inhomogeneous distribution of organic groups inside the pores that severely block the pore entrance due to the incompatibility of silica and organosilica structures. Most of the long chain containing trialkoxy-silsesquioxanes/silsesquioxanes cannot maintain a mesoporous structure after template removal and, being also unstable to the condensation conditions, undergo intramolecular cyclization or simple phase separation from template. When a higher amount of silsesquioxane materials is co-condensed with TEOS totally disordered/amorphous structures are obtained. As a result to control the efficient mesoporous functionalization and to avoid leaching out organic functional groups the amount of functional groups should be in the concentration range of 5–15 mol%.3–5,17,18–37
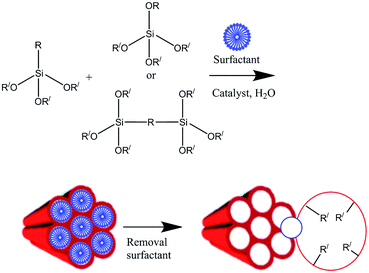 |
| | Fig. 2 Functionalization of mesoporous framework via direct co-condensation method under basic/acidic conditions. OR′ stands for ethoxy/methoxy groups. | |
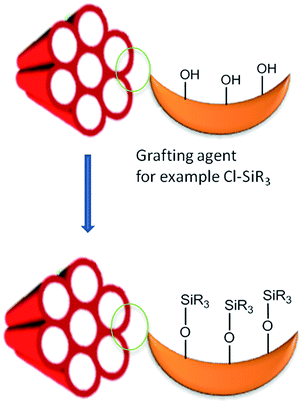 |
| | Fig. 3 Post-functionalization of mesoporous framework via post-grafting method. | |
2.2 Periodic mesostructured organosilicas (PMOs)
Due to the known limitations found on the mesoporous silica framework materials, three independent research groups (Inagaki, Stein and Ozin) in 1999 have reported for the first time the synthesis of PMO hybrids via surfactant-mediated self-assembly process under acid/basic conditions and using simple bridge organosilsesquioxane of the type [(EtO)3Si–R–Si(OEt)3] as sole precursor as shown in Fig. 1.40–52 As can be seen from Fig. 1, the PMOs could be synthesized using bridged silsesquioxanes groups such as [(EtO)3Si–R–Si(OEt)3] as sole precursor, in which R is a functional group with a rigid structure such as ethane/benzene/biphenyl. R in [(EtO)3Si–R–Si(OEt)3] groups should be rigid enough to undergo the surfactant self-assembly process. Afterwards, removing surfactant leaves behind a stable PMO structure with a high surface area along with a composition of [1.5OSi–R–SiO1.5] groups where R is a key organic functional group which creates the flexibility, rigidity, and functionality of the framework of PMOs.20–26,39–94 It is found that the study of bridged polysilsesquioxanes (BP) precedes invention of PMOs with different organic functionalities in the final framework. Bridged polysilsesquioxanes could also be obtained in the form of porous xerogels and aerogels with high surface areas (1000–1500 m2 g−1). Those BPs are highly porous, transparent, and light-weight, and are useful as a matrix for growing metal and semiconductor nanoclusters or for optical applications such as waveguides, lasers, and nonlinear optics.6–11,17–26 The important and key characteristic of a typical PMO synthesis is the use of a hydrolysable organosilane with a bridging organic group instead of a terminal one. The homogeneous distribution of organic and inorganic functions in the final framework of PMOs could easily be ensured whereas organic function is mainly responsible for creating unique advantages of PMOs.6–11,17–26 The organic chemistry of pore walls of PMOs can easily facilitate the introduction of various organic functional groups into the pore channels and provides new opportunities for controlling the chemical, physical, optical, mechanical, surface, dielectric and overall properties of the PMOs. From the prospective point of PMOs, not only the mesoporosity of final PMO structure is what matters. Importantly, the chemical nature of the organic functions in the final PMO structures can also be tailored by the judicious choice of organic bridging groups for targeted applications. In addition, PMOs can reduce catalyst leaching and pore blockage whereas terminal group functionalized mesoporous structures are associated with inhomogeneous distribution of organic functions and leaching out cannot be ruled out during washing/solvent removing. However, as an example, a wide range of different PMOs functionalized with polysilylate molecular organosilica precursors with unique chemical, electrical, and optical properties have been synthesised.20–26,44–104
2.3 Chiral PMOs
Fig. 4 shows different types of chiral organosilane precursors that were used recently to prepare chiral PMOs with new chiral organosilane properties.105–128 As previously reported, chiral organosilane functionalized PMOs showed circular dichroism (CD) or rotation of the polarization of linearly polarized light.110,111 Crudden et al.107 have shown that the synthesis of chiral PMOs based on axially chiral monomers modify the transmission of chirality in the solid state in which the π–π stacking interactions between aromatic systems in the solid state were prominent. A novel bistriethoxysilyl-binapthyl (BINAP) monomer was also co-condensed with a biphenylene-bridged organosilica in the presence of surfactant templates to prepare PMO functionalized with BINAP.107 Complexion of ruthenium followed by asymmetric catalytic hydrogenation and asymmetric transfer hydrogenation were carried out to demonstrate that high levels of activity and selectivity can be achieved with the proposed chiral structure. In addition, it was found that the functional groups could also modify the morphologies and properties of the final product.105–128
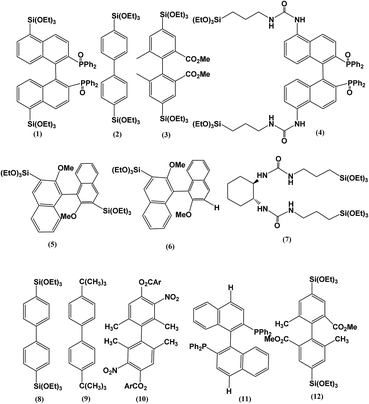 |
| | Fig. 4 Examples of chiral precursors have already been used for producing chiral PMOs, reproduced from ref. 105–107, 124 and 129 with permission from ACS. | |
2.4 Morphology of PMO
Some applications depend on the shape and size of particles of PMOs for example, separation. Meanwhile, PMOs with various shapes including rods, fibres, spheres, gyroid, worm-like, and platelets have been also reported.6–26,39–74 Selection of the synthesis parameters and post-treatment steps for PMOs enables to obtain the desired morphology and shape for a specific applications such as adsorbents, separation, catalysts and optical materials.6–26,39–74 Hole-transport organosilica containing transparent PMO films via evaporation-induced self-assembly process have also been reported for optical and coating applications.59
3. Functional groups directed applications
It is important to note that the nature of organic groups present in mesoporous framework could easily change the functional properties and applications of the PMO structure.6–26,39–84,129–214 PMOs with well-organized pore structures are very suitable for various applications such as heterogeneous catalysis, enzymatic catalysis, enzyme immobilization/adsorption and biomedical devices.6–26,129–214 Meanwhile various studies have shown that the presence of textural properties (pore size and shape, functional groups, surface area and pore volume) in the PMOs are also very significant.6–26 The following sections will focus on various applications/properties with respect to the functional inorganic/organic groups present in the PMOs framework reported since 2012. At the same time, sometimes other important examples will be invoked for the readers.
3.1 Heterogeneous catalysis
In a recent study, lipase-containing PMO particles were used to prepare Pickering emulsions.130 Then the catalytic performance of LP@PE, LP@PMO and native lipase particles were tested for the catalytic esterification reaction of fatty acids into biodiesel using a water fraction 0.65%, molar ratio of ethanol to oleic acid 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, immobilized lipase particles 150 mg, phosphate buffer pH 7.0 and temperature 30 °C. As a result a maximum biodiesel conversion to 95.8% was obtained via esterification of oleic acid with ethanol, whereas the biodiesel yield could be maintained at 88.6% after LP@PE was used 15 times. This significant enhanced activity can be explained due to the considerably increased interfacial area of the Pickering emulsion droplets, which can improve the mass transfer of reactants and the accessibility of enzyme in the reaction system as can be seen in Fig. 5.130
1, immobilized lipase particles 150 mg, phosphate buffer pH 7.0 and temperature 30 °C. As a result a maximum biodiesel conversion to 95.8% was obtained via esterification of oleic acid with ethanol, whereas the biodiesel yield could be maintained at 88.6% after LP@PE was used 15 times. This significant enhanced activity can be explained due to the considerably increased interfacial area of the Pickering emulsion droplets, which can improve the mass transfer of reactants and the accessibility of enzyme in the reaction system as can be seen in Fig. 5.130
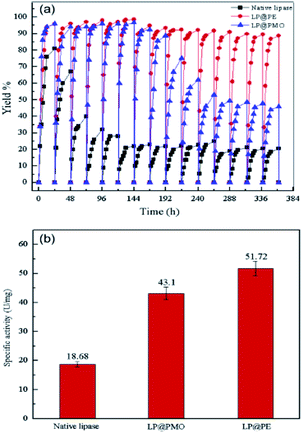 |
| | Fig. 5 The catalytic performance of LP@PE, LP@PMO and native lipase in esterification reactions (the reaction conditions: water fraction 0.65%, oleic acid 0.42 g, molar ratio of ethanol to oleic acid 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, LP@PMO 150 mg, phosphate buffer pH 7.0, 30 °C and 200 rpm). (b) The highest specific activity of LP@PE, LP@PMO and native lipase in esterification reactions, reproduced from ref. 130 with permission from Elsevier. 1, LP@PMO 150 mg, phosphate buffer pH 7.0, 30 °C and 200 rpm). (b) The highest specific activity of LP@PE, LP@PMO and native lipase in esterification reactions, reproduced from ref. 130 with permission from Elsevier. | |
Recently, Karimi et al.131 have also used PMO functionalized with sulfonic acids for biodiesel production. In their study, to optimize the hydrophobic nature of the catalyst in the vicinity of the sulfonic acid groups, they have chosen to incorporate 3-mercaptopropylmethyl dimethoxysilane into the PMO as it is well known that the presence of a methyl group bound to the same silicon atom bearing the sulfonic acid group has a remarkable shielding effect against polar molecules. In another study, it was also reported that ethylene based PMO-PrSO3H catalyst is more active than phenylene based PMO-PrSO3H catalyst during biodiesel formation. It has been suggested that the presence of methyl groups in the PMO may initially prevent that methanol, a polar molecule, react together with reaction by-products such as glycerine and water and subsequently poisoning the active sulfonic acid sites. As a result an increase the catalyst stability was observed that ensure a fast and efficient mass transfer of reactants and products during reaction. In other test, results can be seen in Fig. 6, Karimi et al.131 have compared SBA-15-PrSO3H water tolerance with PMO-PrSO3H showing that by adding 20 wt% water, biodiesel yield decreased by about 50% from its original value on the SBA-15-PrSO3H.
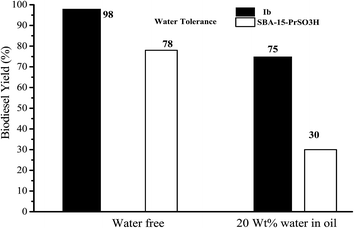 |
| | Fig. 6 Comparing water tolerance test of ethylene-PMO-PrSO3H with SBA-15-PrSO3H (conditions: molar ratio oil/methanol = 1/100, T = 150 °C, 6 and 3 wt% 1b and SBA-15-PrSO3H catalyst, respectively), reproduced from ref. 131 with permission from the RSC. | |
Meanwhile, functionalized mesoporous framework with acidic or basic functional groups or metal-containing catalyst has been investigated for various catalytic applications.20–26,151–164 For example, PMOs with different functional groups such as arene sulfonic acid ethane-silica (AS-MES), mercaptopropyl ethane-silica (Pr-SH-MES) and arene sulfonic acid SBA-15 (AS-SBA-15) were prepared. Then the catalytic reaction of these hybrid PMOs were carried out using the liquid phase acetalization reaction of heptanal by 1-butanol at 75 °C.151 In addition, for comparison, when HY zeolite was used under same condition, it was found lower activity on HY zeolite as compared to the hybrid PMOs,151 due to pore blocking or rapid deactivation of acid sites by water. Among all studied conducted, the higher heptanol conversion observed for arene sulfonic acid ethane-silica (AS-MES) catalyst was the result of the presence of hydrophobic organosilica framework that strengthen it's acid sites on the PMO surface.151 Karimi et al.155 have also demonstrated an example of Ullmann homo-coupling reaction of aryl iodides over a novel recyclable Au nanoparticles catalyst supported on benzene based PMO (Au@PMO). For comparison, Table 1 shows the structure properties of various functionalized PMO samples. In situ reduction of Au3+ with HS-functionalized PMOs creates robust, fine Au nanoparticles and concomitantly produces a sulfonic acid moiety strongly bonded to PMOs.153 It was found that Au nanoparticles with 1.2 nm sizes were mainly attached onto the pore surface rather than onto the outer surface of mesochannels, allowing for maximal exposure to reaction substrates and at the same time minimizing Au nanoparticle leaching.153 The Au-HS-PMO (ethane bridged) or the Au/SO3H-PMO (ethane bridged) produce catalyst with comparably and even higher catalytic efficiency than that of pure PMO. The developed Au-HS/SO3H-PMO(Et) catalyst shows better efficiencies than its homogeneous counterpart in promoting water-medium alkyne hydration, intra-molecular hydroamination, styrene oxidation, and three-component coupling reactions (Table 1). This robust catalyst can easily be recycled and used repetitively at least 10 times without loss of catalytic efficiency.153 At the same time a palladium-containing PMO with alkylimidazolium units has been reported to function as an efficient and reusable catalyst for the Suzuki–Miyaura coupling reaction of various aryl halides in water medium. Similarly ethane-PMO functionalized with a bulky N-heterocyclic carbine moiety has also been found to be efficient materials for Suzuki–Miyaura coupling reaction.156 The Diels–Alder cycloaddition of two common dienes (cyclopentadiene and anthracene) to the double carbon–carbon bonds of an ethenylene-bridged periodic mesoporous organosilica was studied and compared to that of benzocyclobutene (Fig. 7).158 It was found that the double bonds on the walls of an ethenylene-bridged PMO can act as dienophiles for the Diels–Alder reaction when common dienes such as cyclopentadiene and anthracene are used as feed.
Table 1 Structural parameters and catalytic efficiencies in water-medium phenylacetylene hydrationa
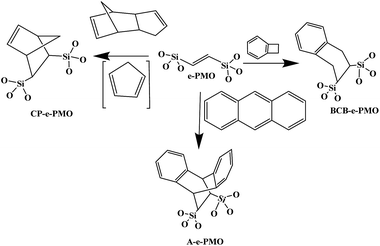 |
| | Fig. 7 Formation of three surface Diels–Alder adducts by reaction of a dienophile (e-PMO) and three different dienes (dicyclopentadiene, benzocyclobutene and anthracene). These units represent a fragment of the organosilica wall and so the oxygens are embedded in the pore wall, reproduced from ref. 158 with permission from the RSC. | |
Similarly, PMOs modified with surface functional groups have shown an ordered mesostructure and thermal stability similar to the parent ethylene-PMO. Ohashi et al.159 have reported a 3-aminopropyltrimethoxysilane (3-APTMOS) grafted benzene-PMO for the Knoevenagel condensation between benzaldehyde and malononitrile. Remarkably, PMOs were found to be more active than pure mesoporous silicas. The same reaction was tested on crystal-like benzene-PMO subjected to a two-step amination process.160 More recently, Hankari et al.161 found that tris(3-(trimethoxysilyl)propyl)amine functionalized PMOs were more active as catalysts for the same Knoevenagel condensation reaction and for the Henry reaction of benzaldehyde and nitroethane. Different types of metal complexes have been also incorporated into the framework of PMOs to be used in heterogeneous green catalytic processes as they are easy to recover, less expensive and non-toxic, making them possible to be used on continuous reaction flow system.20–26,162–171 For example, incorporation of arenetricarbonyl complexes within the organosilica framework of Ph-bridged PMO was successfully reported by a simple chemical vapor deposition (CVD) method.162 Arenetricarbonyl complexes [PhM(CO)3] (M = Cr, Mo) were formed by the reaction of M(CO)6 with the Ph bridges, accompanied by the evolution of CO. Such metal-complex bridged PMOs could easily be employed for the development of unique catalysts such as Ir that catalyses direct C–H borylation of arenes.162 Moreover, it was also reported that PMOs bearing bis-(8-quinolinolato) dioxomolybdenum(VI) complexes can be employed for olefin epoxidation such as cyclooctene in the presence of tert-butyl hydroperoxide (TBHP) as reaction oxidant.171 It is also reported that all MoO2-PMOs catalysts are active for the epoxidation of cyclooctene with nearly 100% of selectivity to epoxycyclooctane. MoO2Q2@PMO-6% has shown the highest conversion of 48.8% after 8 h reaction as a result of its uniform spherical morphology, well defined order and massive mesopores embedded in the framework that facilitate the mass transfer of reactant and products. It was found that catalyst is stable instead of significance leaching.171 On the other hand, well-organized metal-tetrakis(carboxyphenyl)porphyrin (metal = Fe, Cu, Sn) bridged PMOs (M-TCPP-PMO) with high surface area were synthesized and used as catalyst for the Baeyer–Villiger oxidation of ketones.168 Among the catalysts, the high catalytic activity observed for Fe-TCPP-PMO over Cu-TCPP-PMO and Sn-TCPP-PMO was attributed to the high Fe-porphyrin valence on the pore wall of organosilica framework along with cyclohexanone, suggesting that Fe-TCPP-PMO could be a promising and efficient catalyst for the Baeyer–Villiger oxidation of ketones to esters using O2 as the oxidant.168 Importantly, no leaching was observed when vanadyl Schiff base complexes were successfully employed to catalyze efficiently the cyanosilylation of carbonyl groups.163 Bridge organophosphine complexes functionalized PMOs have been also reported as catalysts for a great variety of organic reactions (such as Suzuki cross-coupling, Diels–Alder) and different bis- and tris-silylated phosphine complex precursors in the presence of various different metals.164,166,169–171 These materials have shown similar/very comparable activities to the homogeneous complexes and could easily be recycled few times due to their resistance to leaching. For example, co-condensed PMOs were found to be more active than similar grafted materials because the grafting method could lead to pore blockage and leach out catalyst during washing time.4,5,15 Recently, Zhang et al.46 has reported that immobilization of a site-isolated organopalladium functionality and chiral organoruthenium functionality on a single PMO was used for synthesis of chiral biaryl alcohols by cascade asymmetric transfer hydrogenation and Suzuki cross-coupling of haloacetophenones and arylboronic acids (Fig. 8).46
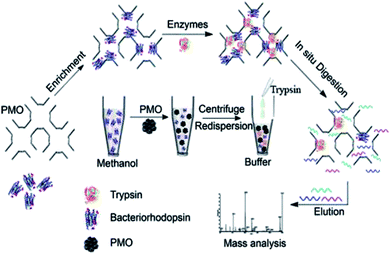 |
| | Fig. 8 Schematic Illustration of the PMO-assisted strategy employed for efficient identification of hydrophobic membrane proteins, reproduced from ref. 174 with permission from ACS. | |
3.2 Enzyme immobilization
PMOs are also being used as host materials for adsorption/immobilization of biomolecules.20–26,132–148 Recently a hydrophobic benzene based PMO material has shown better adsorption affinity to lysozyme when compared with a ethane-based PMOs.132 It is known that ordered mesoporous structured materials with rather narrow pore sizes are excellent supports for lipase immobilization when the surface is more hydrophobic. This is not possible via grafting with organic functional groups because post-grafting method is not only highly associated with leaching out organic functional groups but also with pore blocking and heterogeneous distribution of organic functional groups. What happens is that the soft interaction of the protein with the hydrophobic ungrafted surface via post-grafting method results in a much lesser activity loss.133 It was found that horseradish peroxidase (HRP) can also be immobilized on PMOs. Studies suggested that the organic–inorganic nature of framework gives them superior properties when used as supports, as could also protect immobilized enzyme and enhance its enzymatic activity.132–148 Recently a monodisperse nanosize Co3O4 core-mesoporous organosilica shell nanosphere was synthesized by cooperative self-assembly of organosilica precursor and surfactant molecules on the Co3O4 nanoparticles surface. Co3O4/PMO core–shell structured nanospheres are highly promising to the adsorption of microperoxidase-11 (MP-11).146 More excitingly, it was found that core–shell nanospheres structures have a positive effect on the catalytic reaction of microperoxidase-11 (MP-11). Lu et al.147 have coated and encapsulated barium ferrite BaFe12O19 (BaFeO) nanoparticles (with a size of 100 nm) inside PMO host material, forming magnetic PMO nanoparticles with a relatively high magnetic response (25 emu g−1) and a high loading capacity of 1.315 mg per mg towards enzyme mixture. This study further demonstrated that MHPMO materials exhibited enhanced cellulose tissue penetration behaviour under external magnetic field.147
3.3 Refolding of proteins and protein membrane
Many studies have examined applications of PMOs in the field of adsorption and delivery of drugs, genes and enzymes under a range of internal/external stimuli, such as temperature, redox, pH, light, magnetism and ultrasound,20–26,172–174 whereas an interesting application of PMOs for protein refolding has not been sufficiently explored yet.173 Feng et al.172 reported that ethylene-bridged PMOs with pore size 4.8 nm effectively entrap unfolded proteins and assist refolding by controlled release into the refolding buffer. Hen egg white lysozyme (molecular dimensions of 1.9 × 2.5 × 4.3 nm3) was used as a model protein to demonstrate the new method of protein refolding and how the organized mesopores of PMO can effectively entrap denatured proteins and minimize protein aggregation.172 This study has suggested that ethane-PMOs with surface silanols can interact with hydrophobic parts of the protein and the exposed charge of amino acid residues, respectively, which contributes to adsorption of the protein. This has also shown that poly(ethyleneglycol) (PEG)-triggered continuous release of entrapped denatured lysozyme that allows high yield refolding with high cumulative protein concentrations. This new method enhances the oxidative refolding of lysozyme (e.g., over 80% refolding yield at about 0.6 mg mL−1).172 Moreover, the PEG molecules can form strong hydrogen bonds with the Si–OH groups. It was found that when comparing with SBA-15 samples the release is much more hindered in comparison to the PMO samples that give the best release velocity results. Gan et al.174 have developed a versatile protocol shown in Fig. 9 for large-scale characterization of membrane proteins using a three-dimensional PMO-based membrane nanodevice for detecting proteins at a low concentration. This study shows an effective method to the digestion of complex samples indicating its potential application in membrane proteome analysis.
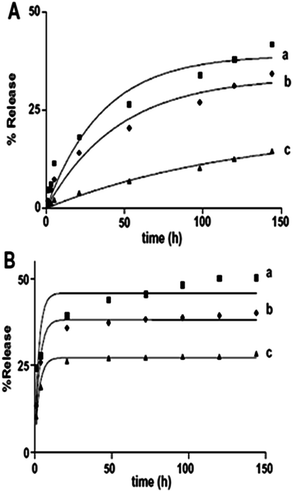 |
| | Fig. 9 Release kinetics of TC loaded on PMS-SS (a), PMO-SS (b), PMO-HS (c) in SBF solution (A) and PB solution (B) respectively. Symbols: experimental data; solid lines: fitting results by first order mass transfer model, reproduced from ref. 150 with permission from Elsevier. | |
3.4 Biomedical applications (drug delivery and biomedicine)
Application of PMOs also largely depends on how and what kind of organic functional groups are integrated into the framework of PMO.20–28,157 Lu and co-workers have reported the effects of morphology, wall composition and the use of different buffer solutions on drug delivery application and how these properties have played an important role in controlling both adsorption capacity and the release rate of drug molecules.150 In this way the authors found that hybrid PMO materials with hydrophobic ethylene group on their wall framework possess higher loading capacities and significantly lower release rate of tetracycline (TC) than pure PMS materials because of their stronger hydrophobic–hydrophobic van de Waals attraction forces with tetracycline molecules. Two kinds of release medium that were used in this study i.e., simulated body fluid (SBF) solution (pH 7.4) and phosphate buffer (PB) solution (pH 1.5), revealed very different release profiles. A slower delivery rate was observed when SBF solution was used that can be attributed to the different ionic interactions between the guest molecule and the host material in the two different pH solutions. Overall, hollow PMO shows the lowest release rate and the highest loading amount compared to the other two materials (Fig. 9).150 Recently, it has been reported that PMO with hetero-aromatic pyridine ring containing two N–H sites showed few advantages including good mesostructural order, selective nucleobase-recognition properties, and stimuli-responsive site-specific delivery of anticancer agents to cancer tissues into a single entity.149 Fig. 10 shows the time-dependant release profiles of 5-FU from DMPy-PMO-20 (N,N′-disilylated pyridine-bridged PMOs) at pH 7.4, 6.2, 5.0, and 4.0 in PBS buffer medium. From the result it can be assumed that this hybrid PMO chemo-sensor showed an intrinsic selective recognition for a nucleobase, such asthymidine, due to multipoint hydrogen-bonding interactions that comprises of suitable arrayed receptor sites loaded into the rigid silica framework. An in vitro cytotoxicity test suggested the good biocompatibility of designed chemo-sensor materials (Fig. 11) and, therefore, they could be promising potential candidates for the delivery of a range of therapeutic agents. In addition, confocal laser scanning microscopy (CLSM) confirmed that the material can be internalized effectively by cancer cells (MCF-7 cells).149
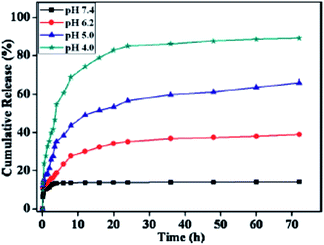 |
| | Fig. 10 The time-dependant release profiles of 5-FU from DMPy-PMO-20 at pH 7.4, 6.2, 5.0, and 4.0 in PBS buffer medium, reproduced from ref. 149 with permission from Wiley. | |
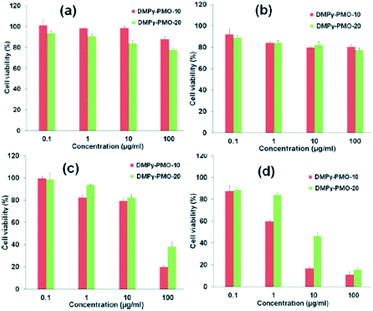 |
| | Fig. 11 The in vitro cytotoxicity of DMPy-PMOs with two different particle sizes with various functional-group contents against MCF-7 cells at pH 7.4 and 6.1. Cytotoxicity profiles of blank DMPy-PMO particles (a and b) and 5-FU-loaded DMPy-PMO particles (c and d) at concentrations of 0.1, 1, 10, and 100 μg mL−1 at pH 7.4 (a and b) and 6.1 (c and d), reproduced from ref. 149 with permission from Wiley. | |
In 2014, Wang et al.157 designed molecularly organized hollow PMO nanoparticles (HMONs) with a variety of silsesquioxanes bridged to demonstrate how these materials can improve therapeutic performance and enhance the biological effects beneficial for biomedicine application. Fig. 12 shows their strategy which was adopted using a generic intelligent framework-hybridization approach for biomedicine.157 They found that the hybridization of physiologically active thioether groups with triple distinctive disulphide bonds present in the framework structure can provide HMONs with unique intrinsic reducing/acidic and as such external high intensity focused ultrasound (HIFU)-responsive drug-releasing performances, improved biological effects (e.g., lowered hemolytic effect and improved histocompatibility), and enhanced ultrasonography behavior.157 The doxorubicin-loaded HMONs with concurrent thioether and phenylene hybridization exhibit drastically enhanced therapeutic efficiency against cancer growth and metastasis, as demonstrated both in vitro and in vivo. This study showed that that the triple disulfide bonds in a thioether group within the framework are highly responsive to reducing environments to quickly trigger the releasing of loaded guest molecules. The releasing patterns of Dox-loaded HMONs in Fig. 13 were studied at different GSH (0, 5, and 10 mM) concentrations in a phosphate buffer solution (PBS). The release of Dox in pure PBS showed slow and sustained profile with only 21.8% releasing percentage in 24 h whereas the addition of GSH has increased releasing percentage to 47.2% and 69.0% at the GSH concentrations of 5 and 10 mM, respectively, in 24 h. It can be concluded that in this hybrid system, after the reduction and subsequent cleavage of disulfide bonds within the thioether functional groups, the initially established π–π supramolecular stacking and hydrophobic–hydrophobic interaction between the framework and the aromatic Dox molecules can be broken up, leading to the substantially increased drug-releasing rates.157
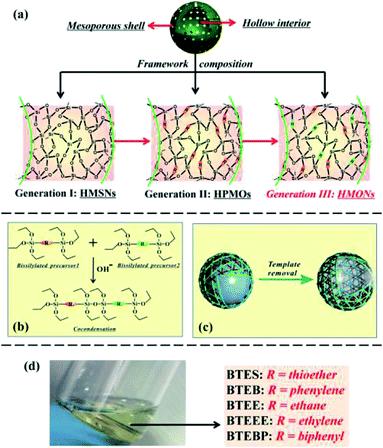 |
| | Fig. 12 (a) Illustration of the evolution of three generations of silica-based hollow mesoporous capsules classified in terms of chemical composition of the shell. (b) The chemical homology principle for constructing the hybrid silsesquioxane framework of HMONs. (c) The scheme of templating method to fabricate dual-functionalized HMONs (R1: thioether, R2: phenylene). (d) Photographic image of homogeneous solution of up to five bissilylated organosilica precursors miscible with each other, including BTES (R1), BTEB (R2), BTEEE (R3), BTEE (R4), and BTEBP (R5), reproduced from ref. 157 with permission from ACS. | |
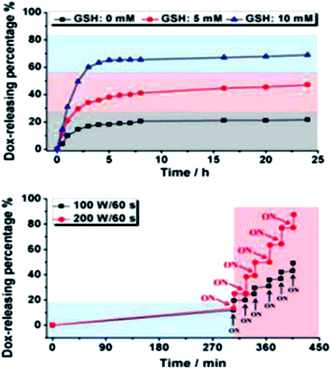 |
| | Fig. 13 (Top) Cumulative Dox-releasing percentages at the glutathione (GSH) concentrations of 0, 5, and 10 mM in PBS solutions. (bottom) HIFU-triggered Dox-releasing profiles at different power densities (100 and 200 W cm−2, 60 s irradiation), reproduced from ref. 157 with permission from ACS. | |
Fig. 14 shows also the therapeutic efficiency of HMONs for in vivo anticancer drug delivery/chemotherapy assessed on 4T1 orthotopic mammary tumor spontaneous metastasis xenograft. It is suggested that the tumoridical efficiency of Dox loaded HMONs is significantly higher than free Dox (Fig. 14a). The tumor volume (Fig. 14b) and weight (Fig. 14c) were found to be reduced for Dox-HMONs treated samples compared with those of free Dox group. In addition, the high tumoridical efficiency by Dox-HMONs could be supported by the fact that significantly enhanced tumor destruction and cell apoptosis of Dox-HMONs group has been reported by pathological hematoxylin and eosin stain (H&E) analysis and TUNEL staining.157 It could be suggested that one of the studies that shows the delivery of Dox mediated by HMONs can bring substantially enhanced destruction/apoptosis effects against tumors, significantly inhibited tumor metastasis and decreased angiogenesis.157
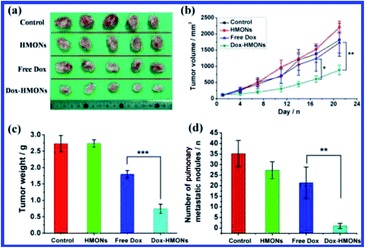 |
| | Fig. 14 (a) Photographic images of tumors after different treatments. (b) The tumour volume changes as a function of time after different treatments (n = 5). (c) The tumor weights at the end of treatment. (d) The number of pulmonary metastatic nodules in lungs of mice after different treatments. **P < 0.01, ***P < 0.001, reproduced from ref. 157 with permission from ACS. | |
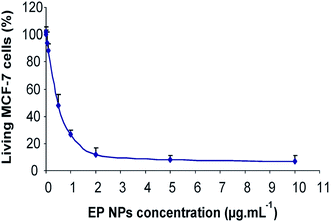
On another interesting work, Durand et al.140 have described the synthesis of new mixed PMO nanoparticles (MPMO NPs) as a result of the co-condensation product of a tetra-trialkoxysilylated two photon photosensitizer with bis-(triethoxysilyl)phenylene or ethylene. The MPMO NPs are found to be very efficient for anti-cancer drug delivery combined with two-photon therapy in MCF-7 breast cancer cells, leading down to 76% cancer cell death. This study has used a series of PMOs with different conditions and found that MPMO NPs are very promising for nanomedicine applications. Same group has reported porphyrin-functionalized ethylene bridged PMO nanoparticles (denoted at EP-NPs) for two-photon imaging of breast cancer cells and drug delivery applications.137 The two-photon imaging capacity of the EP-NPs assessed in cancer cells is shown in Fig. 15. Finding from this study has demonstrated the suitability of porphyrin-functionalized mesoporous organosilica nanoparticles for two-photon imaging. In addition to two-photon fluorescence study, EP NPs were incubated with MCF-7 for 72 h and found to be remarkably efficient to cause cell death as only 25% of cancer cells survived at a concentration of EP NPs of only 1 μg mL−1 (Fig. 16).
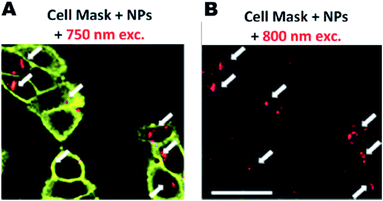 |
| | Fig. 15 Cell membranes were stained with Cell Mask™ orange plasma membrane, EP NPs were incubated and irradiated at 750 (A) and 800 nm (B). Arrows indicate NPs in cells. Scale bar is 10 mm, reproduced from ref. 137 with permission from the RSC. | |
 |
| | Fig. 16 Doxorubicin delivery in MCF-7 cancer cells via EP NPs, reproduced from ref. 137 with permission from the RSC. | |
3.5 Magnetic PMOs
The modification or coating of magnetic nanoparticles with silica or polymers has been extensively studied for biomedical fields including targeted drug delivery systems, magnetic resonance imaging (MRI) contrast agents, protein separations, cell sorting, and immunoassays.177–183 Recently, Lu and his co-workers182 have demonstrated a novel class of magnetic composite PMO hollow spheres and Fe3O4 nanocrystals. The high saturation magnetization ensures (Fig. 17) that these functional mesoporous hollow spheres can be used as a very efficient protocol to targeted drug delivery and also be assisted by magnetic fields. In addition, the principle demonstrated in this work may also allow core/shell synthesis of nanocrystals or semiconductor quantum dots (e.g., CdSe, Au) encapsulated in hollow silica spheres, which may lead to many promising applications in enzyme immobilization, cancer therapies, and MRI contrast agents but toxicity studies of these suggested quantum dots have to be ensured for before practical applications.
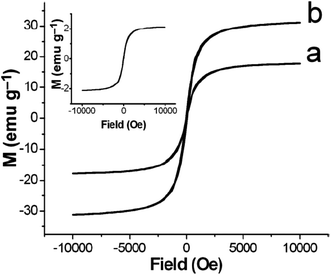 |
| | Fig. 17 Field-dependent magnetization curves at 300 K of (a) PMO–MHS2, and (b) PMO–MHS4. Inset: curve for PMO–MHS1, reproduced from ref. 182 with permission from Wiley. | |
3.6 Chromatography
Chromatography is currently the most commonly applied technique for separating and analysing multi-component mixtures.20–26,184–195 In this field, silica is commonly used as stationary phase whereas more than 60% of all HPLC separations are carried out under reversed phase (RP) conditions.184–186 The surface modification of well-defined silica beads with T-silyl functionalized organic systems is the major route for creating phases for reversed phase liquid chromatography (RPLC). In this context, the presence of organic and inorganic functions in PMO framework can tune the polarity of the PMOs and as such it could be easier to design a whole broad spectrum of stationary phases which can be located in every desired place between the extremes of a normal (very polar pure silica phase) and a reversed HPLC phase (very non-polar phase consisted of silica capped with organic hydrophobic group). Corriu et al.186 have shown that PMOs with high surface areas along with particle size less than 1 μm have been used as suitable stationary phases in HPLC for separating different standard reference materials (SRMs) containing polycyclic aromatic hydrocarbons (Fig. 18). It was found that the spherical phenylene-PMOs (0.16 silanol groups per nm2) were able to cleanly separate all three test mixtures (BHT, sorbic acid, and aspartame), despite the wide range of polarities of the individual analyte when compared with a normal phase Nucleosil 50-10 (Nucleosil 50-10 contains a significantly higher concentration of 4.82 silanol groups per nm2).187 Phenyl-functionalized ethane-bridge PMOs have shown better separation capacity of polycyclic aromatic hydrocarbons than C8-immobilized pure mesoporous silicas. The chiral PMOs with trans-(1R,2R)-diaminocyclohexane group have higher enantioselectivity for nine amino acids over other chiral silica based stationary phases and these chiral PMOs with trans-(1R,2R)-bis-(ureido)-cyclohexane also provide baseline separation of (R/S)-1,1′-bi-2-naphthol enantiomers at high sample loading and high flow rate.194,195 Recently, Ide et al.185 have reported the synthesis of a novel cyclic multifunctional organosilane-based PMO with 2 μm size particles. The PMO spherical silica particles are not only hydrolytically stable stationary phase in reversed phase HPLC but also proved to be both highly retentive and stable in a pH range from 1.75 to 12 and up to 150 °C in 60 v% water, indicated the high stability of the backbone and the functionalities of the material.
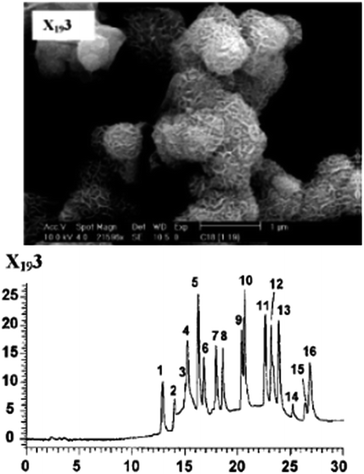 |
| | Fig. 18 (Top) SEM image of X193 and (Bottom) separation of the 16 priority pollutant polycyclic aromatic hydrocarbons (SRM1647c: (1) naphthalene, (2) acenaphthylene, (3) acenaphthene, (4) fluorene, (5) phenanthrene, (6) anthracene, (7) fluoranthene, (8) pyrene, (9) benzo[a]anthracene, (10) chrysene, (11) benzo[b]fluoranthene, (12) benzo[k]fluoranthene, (13) benzo[a]-pyrene, (14) dibenz[a,h]anthracene, (15) benzo[ghi]perylene, (16) indeno[1,2,3-cd]pyrene on the mesoporous stationary phases X193, reproduced from ref. 186 with permission from Wiley. | |
3.7 Environmental (inorganic and organic pollutants)
PMOs can play also very significant role for water treatment process and environmental applications since functionalized PMOs could be in the range of materials with targeted functional groups that could be used efficiently and according to target pollutants.20–26,196–201 For example, ethylenediamine ligand of N,N′-bis[3-(trimethoxysilyl)propyl]-ethylenediamine an important part of PMO framework enables to bind metals directly within the framework walls of PMOs (Fig. 19).197 On the other hand, crystalline PMOs with densely packed pyridine units (v-Py–PMO) within the framework were found to be accessible for reversible protonation and Cu2+ adsorption.198 In comparison, pyridine groups containing PMOs show higher adsorption capacity for Cu2+ whereas similar PMOs with divinylbenzene group (Si–C2H2–C6H4–C2H2–Si) showed little Cu2+ adsorption capacity.170 The adsorption capacity, 2.4 mmol g−1 at 0.018 M Cu2+, indicated that 71% of the total pyridine groups in v-Py–PMO are bound with Cu2+. The adsorbed Cu2+ was almost completely removed by washing with water while preserving the PMO structure. On another example, PMOs functionalized with isocyanurate groups showed an adsorption capacity of 1.8 g of Hg2+ per g of adsorbent199 whereas disulfide groups functionalized PMOs have an adsorption capacity of the same material of about 864 mg g−1 of Hg2+ ions, demonstrating its potential use as an adsorbent for adsorption of heavy metal ions from wastewater. Recently a synthesized PMO containing the hetero-aromatic pyridine ring and two N–H sites in the framework showed adsorption capacity of 0.95 mmol g−1 for Cu2+.149 The enhanced adsorption capacity of functionalized PMOs was due to the presence of electron-rich pyridine and N–H sites. Beside these, depending on the nature of organic ligands in the framework of PMOs, a broad wide range of PMOs with various organic groups have been developed and employed for studying the adsorption capacity of heavy metal ions including Cu2+, Cd2+, Cr3+, Hg2+, Mn2+, Fe3+, Fe2+, Co2+, Ni2+, Zn2+, Pb2+, Pd2+, Pt2+, Au3+.20–26,196–206 Recently, PMOs with benzene and ethane groups were also used for the selective adsorption of organic pollutants including 2,4,6-trinitrotoluene (TNT), cresol, 4-nitrophenol, phenol, 1,3,5-hexahydro-1,3,5-trinitrohydrazine and benzene.203–206 It was found that the hydrophobic nature of the PMOs themselves imparted good sorption properties towards these compounds (up to 500 mg g−1). Depending on the nature of functional groups in the PMO framework, these PMOs could be used for adsorption of toxic oxyanions, rare earth metal ions, radionuclides, dyes, pesticides and pharmaceuticals.
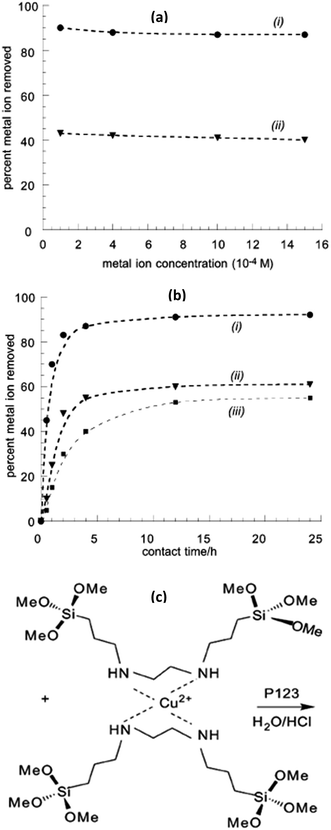 |
| | Fig. 19 (a) Cu2+ and Zn2+ uptake by EBPEDS-0.2 (solutions equimolar in Cu2+ and Zn2+). Samples are ethanol/HCl extracted, and rinsed with dilute NH4OH and water prior to equilibration for 4 h. (b) Percent copper absorbed from aqueous solutions (10−3 mol dm−3) of the corresponding acetate versus contact time for (i, iii) Cu2+; (ii) Zn2+; (i, ii) samples rinsed with NH4OH and water prior to uptake experiment; (iii) sample in protonated form. (c) The synthesis of bridge-bonded bifunctional PMO towards metal on, reproduced from ref. 197 with permission from ACS. | |
3.8 Sensors
A sensor is a device that detects and responds to some type of input from the physical environment. The specific input could be light, heat, motion, moisture, pressure, or any one of a great number of other environmental phenomena. The output is generally a signal that is converted to human-readable display at the sensor location or transmitted electronically over a network for reading or further processing.20–26,112–207
PMOs with organized pores have attracted attention as potential sensor devices because mesoporous channels allow incorporation of guest compounds into the mesoporous framework and might possess suitable interaction centres in their interior/pore walls structures, which may then induce a signal, i.e. a property change that can be detected. Recently, Crudden et al.207 have reported PMO coating as the key component in a sensitive and tunable fiber-optic evanescent wave sensor for the ppb-level detection of Pb(II) (Fig. 20). Authors have suggested the possibility of extending this method to detect industrially relevant metal contaminants including mercury and nickel. Importantly, they also stressed that such chemically selective, processable, and versatile mesoporous organosilica films will be of great interest to research as have the potential to develop micro-resonators and other sensitive optical and evanescent field devices. Detection of organic explosive such as p-nitrophenol, p-cresol, 2,4,6-trinitrotoluene and RDX (1,3,5-trinitroperhydro-1,3,5-triazine) using porphyrin embedded nanoporous organosilicas has recently been successfully reported.210 Ha et al.209 used rare-earth ions (Eu3+, Tb3+ and Tm3+) to dope PMO monoliths which have shown red-, green- and blue-emissions upon UV irradiation with characteristic emission peaks, that have also been suggested for UV-sensors. In 2015, Qiu et al.212 have demonstrated pentyl-linked bis(rhodamine)-derived tetra-siloxane (PRh-Si4) based highly ordered PRh-bridged periodic mesoporous organosilicas (PRhPMOs) for ratiometric fluorescent nanosensors for copper(II). Fig. 21 shows the UV/Vis spectra of PRh-Si4 and PRhPMO-10 before and after the addition of copper ions. It is reported that a band centred at λ = 294 nm and a band at 528 nm emerged for PRh-Si4 upon the addition of Cu2+, which is the result of the ring opening of the five-membered nitrogen-containing heterocycle in the PRh unit after complexation whereas the π–π* electronic-transition occurred due to the aromatic rings in the framework. A broad red shifted peak at λ = 600 nm was found for Cu2+-chelated complex PRhPMO-10 due to the formation of J-aggregates of the PRh units in PRhPMO-10. The presented results suggested the possibility to use PRhPMO ratio metric fluorescent chemosensors for Cu2+.212
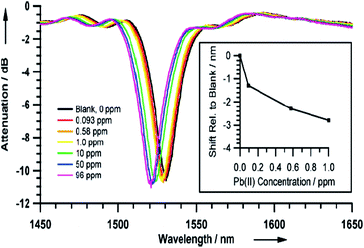 |
| | Fig. 20 Optical response of the PMO-coated LPG to aqueous DMSO solutions (RI1/41.402) of Pb(II) of increasing concentration as observed with the LPG attenuation spectra and by the shift in the resonant peak position as a function of solution concentration (inset), reproduced from ref. 207 with permission from Wiley. | |
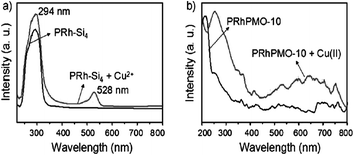 |
| | Fig. 21 (a) UV/Vis spectra of PRh-Si4 in THF before (PRh-Si4) and after (PRh-Si4+ Cu2+) the addition of copper ions. (b) UV/Vis diffuse reflectance spectra of PRhPMO-10 and Cu2+-chelated PRhPMO-10, reproduced from ref. 212 with permission from Wiley. | |
3.9 Gas (adsorption/separation) and hydrogen storage
Gas separation/adsorption/storage processes are very important and being explored in terms of academic and practical environmental and industrial discharge.118–212 Hence, ethane-bridged PMO materials have been used for the adsorption of various gases including sulfur dioxide, cyanogen chloride, ammonia and octane (Fig. 22). Systematic studies on the adsorption of hydrogen gas and its interactions with the various bridged organic functional groups (ethylene-, ethane-, phenylene- and biphenylene)-based PMO materials have been explored, whereas it was found that ordered phenyl-PMOs can adsorb more hydrogen than the ethane-PMOs.216 On the other hand, phenyl-PMO was used to remove highly toxic polycyclic hydrocarbons from aqueous solutions217–219 whereas functionalized amine-bridged PMO materials are found to be efficient for CO2 adsorption. The low-temperature interaction of CO with the hybrid surface of crystalline phenyl PMOs has been proven as a result of the double interaction that arises from dispersive interaction between the CO molecule and the polarizable benzene ring.220 Very recently, ordered and disordered phenylene-PMOs have shown properties as materials for H2 storage.221 These materials exhibit a reversible hydrogen excess surface adsorption capacity up to 2.10 wt% at 6 MPa and 77 K. DFT calculations that were performed to define the binding strength of H2 with the pore walls found an interaction energy value of 0.55 kcal mol−1, higher than the corresponding interaction energy value of hydrogen with a single benzene molecule.221
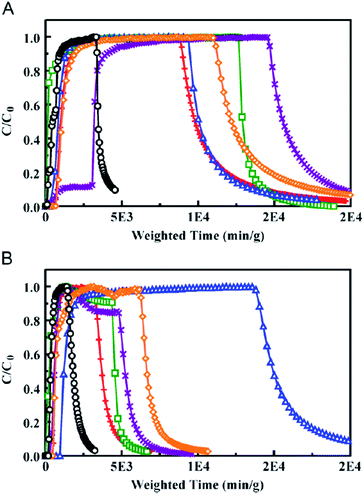 |
| | Fig. 22 Ammonia micro breakthrough curves for organosilicates under (A) dry and (B) humid RH (80% RH) conditions. E50 as synthesized (X), at pH 7 (diamond), and pH 9 (+) is compared to BPL/Zn/TEDA (circle), E50-A (square), and E50-I functionalized (triangle) materials. Overall, the material grafted with isocyanate functional groups (E50-I) exhibited the best ammonia removal capabilities, reproduced from ref. 215 with permission from Elsevier. | |
3.10 Light harvesting (LH) materials based PMOs and photocatalysis
The use of energy harvesting materials have received vast interest since energy based applications as photocatalysts, photovoltaic devices, and light-emitting devices will play important role in the future energy sectors. Recently, the light-harvesting effects of PMOs that contain a high density of aromatic bridges has been explored successfully since aromatic chromophores could easily be located in the framework and meso-channels of PMOs, enabling the rational design of excited energy transfer from donors in the framework to acceptors in the meso-channels. The tuning of PMO pore walls with different photoactive species enables the energy transfer between two species within a single material.22,222–233 Recently, biphenyl-bridged hexagonal PMO with a crystal-like pore wall structure was used as a template to build two- and three-chromophore systems with a light-harvesting function as shown in Fig. 23.223 Table 2 shows the summary of the synthesized materials with the energy transfer efficiency. Two-chromophore systems with an additional dye in the pores show that excitation energy absorbed by biphenyl groups (donors) can be transferred to either aminoacridone or diethylaminocoumarin (acceptors) through FRET with high transfer efficiencies of 91–95%.223 On the other hand, light-harvesting three-chromophore systems were prepared by incorporation of two types of dye molecules into biphenyl-bridged PMO, in which each dye in the mesochannels acts as a mediator and acceptor. The consecutive two-step-FRET from biphenyl groups to acceptors, conveyed by mediators is observed with high energy-transfer efficiencies from 70 to 80% at very low concentrations of each dye. Results have suggested that the organized pores of PMO contain enabled mediator/acceptor pairs that can be arranged in close mutual proximity for an efficient direct energy transfer.223 Inagaki et al.227 have also reported that the increasing amount of light harvesting coumarin doped-PMOs weakened fluorescence emission of biphenyl group whereas the blue emission of coumarin dye strengthened it. The energy transfer efficiency estimated from the fluorescence quenching of the biphenyl bridge is close to 100%, even at a low coumarin concentration of 0.80 mol% to the 4,40-biphenylylene (Bph) bridged unit (one coumarin molecule per 125 Bph units).227 As shown in Fig. 24, the fine-tuning of the emission colors over a wide range of the visible spectrum including a white light emission was reported for fluorescence dyes doped-PMO, indicating possible luminescence applications including LEDs and fluorescence sensing.229 Evaporation-induced self-assembled transparent and visible light-harvesting acridone-bridged containing PMO films (from non-methylated and N-methylated acridonebridged bis-triethoxysilane precursors) have shown that non-methylated precursor influenced the opaque and aggregated films as result of the presence of intermolecular hydrogen bonds between N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O on the acridone groups whereas N-methylated precursor functionalized PMO films were transparent, suggesting that capping of the amine group hindered the intermolecular hydrogen bonds and effectively suppressed aggregate formation during PMO films casting.228 Uvdal et al.232 have reported that ligands with different p-conjugation lengths and side chains show impact on the optical properties. Direct modification of the ligands by using hydrophobic alkyl chains has been proven to effectively stabilize the particles. Different metal ions, donors, and acceptors have been incorporated into the frameworks by one-step coordination-directed assembly. Experimental results clearly show that the optical properties of nanoscale light-harvesting metal–organic frameworks (NMOFs) can be enhanced and tuned by chemical manipulation of the inorganic and organic components in the frameworks. The development of photocatalytic systems linked with light-harvesting function is also important.234–240 Hirai et al.237 have immobilized CdS nanoparticles into PMOs to be used as photocatalyst for the generation of H2 from 2-propanol aqueous solution. It was shown that morphology, stability, and framework hydrophobicity have affected photocatalytic activity. On the other hand, Ti-functionalized PMO (Ti-PMO) was also found to be employed as a photocatalyst for selective olefin epoxidation.210 The reaction rate on the Ti-containing PMO catalysts is similar to that on Ti-containing pure silica due to the destabilization of these oxygen radicals on the hydrophobic surface of the Ti-PMO catalysts. It is well-known that the O3− radical is a crucial oxidant for epoxide formation which is more destabilized once O3− radical is on the hydrophobic Ti-PMO surface. On the other hand, the hydrophobic olefins also easily access the photoexcited Ti–O4 species on the hydrophobic Ti-PMOs surface. What happened then is that the destabilizations of the photoexcited Ti–O4 species and O3− radical counteract the enhanced access of olefins to the excited species, resulting in almost no improvement in the olefin conversion.238 In 2014, a visible-light harvesting antenna based on acridone groups functionalized PMO (Acd-PMO) was successfully synthesised using [RuII(bpy)32+] complex (bpy = 2,2′-bipyridyl) coupled with iridium oxide (IrOx) particles supported in the PMO meso channels framework as photosensitizer and catalyst, respectively.239 It was found that Acd-PMO absorbed visible light and funnelled the light energy into the Ru complex on the meso channels through excitation energy transfer. From this study, the reaction quantum yield was 0.34%, which was improved to 0.68% by the modifications of PMO.239 Inagaki and co-workers239 also reported surfactant-mediated PMO for an artificial photosynthesis system with three-dimensional organization of molecular parts, such as a light absorber and a Re-complex as the multi-electron catalyst. A rhenium (Re) bipyridine complex was chosen as a reaction centre as is a well-known two-electron-reduction photocatalyst for conversion of CO2 to CO. This is a very new conceptual design for enhancing the photocatalytic CO2 reduction of rhenium(I) complex placed in the mesochannels of PMO framework.
O on the acridone groups whereas N-methylated precursor functionalized PMO films were transparent, suggesting that capping of the amine group hindered the intermolecular hydrogen bonds and effectively suppressed aggregate formation during PMO films casting.228 Uvdal et al.232 have reported that ligands with different p-conjugation lengths and side chains show impact on the optical properties. Direct modification of the ligands by using hydrophobic alkyl chains has been proven to effectively stabilize the particles. Different metal ions, donors, and acceptors have been incorporated into the frameworks by one-step coordination-directed assembly. Experimental results clearly show that the optical properties of nanoscale light-harvesting metal–organic frameworks (NMOFs) can be enhanced and tuned by chemical manipulation of the inorganic and organic components in the frameworks. The development of photocatalytic systems linked with light-harvesting function is also important.234–240 Hirai et al.237 have immobilized CdS nanoparticles into PMOs to be used as photocatalyst for the generation of H2 from 2-propanol aqueous solution. It was shown that morphology, stability, and framework hydrophobicity have affected photocatalytic activity. On the other hand, Ti-functionalized PMO (Ti-PMO) was also found to be employed as a photocatalyst for selective olefin epoxidation.210 The reaction rate on the Ti-containing PMO catalysts is similar to that on Ti-containing pure silica due to the destabilization of these oxygen radicals on the hydrophobic surface of the Ti-PMO catalysts. It is well-known that the O3− radical is a crucial oxidant for epoxide formation which is more destabilized once O3− radical is on the hydrophobic Ti-PMO surface. On the other hand, the hydrophobic olefins also easily access the photoexcited Ti–O4 species on the hydrophobic Ti-PMOs surface. What happened then is that the destabilizations of the photoexcited Ti–O4 species and O3− radical counteract the enhanced access of olefins to the excited species, resulting in almost no improvement in the olefin conversion.238 In 2014, a visible-light harvesting antenna based on acridone groups functionalized PMO (Acd-PMO) was successfully synthesised using [RuII(bpy)32+] complex (bpy = 2,2′-bipyridyl) coupled with iridium oxide (IrOx) particles supported in the PMO meso channels framework as photosensitizer and catalyst, respectively.239 It was found that Acd-PMO absorbed visible light and funnelled the light energy into the Ru complex on the meso channels through excitation energy transfer. From this study, the reaction quantum yield was 0.34%, which was improved to 0.68% by the modifications of PMO.239 Inagaki and co-workers239 also reported surfactant-mediated PMO for an artificial photosynthesis system with three-dimensional organization of molecular parts, such as a light absorber and a Re-complex as the multi-electron catalyst. A rhenium (Re) bipyridine complex was chosen as a reaction centre as is a well-known two-electron-reduction photocatalyst for conversion of CO2 to CO. This is a very new conceptual design for enhancing the photocatalytic CO2 reduction of rhenium(I) complex placed in the mesochannels of PMO framework.
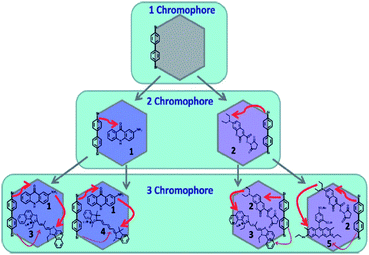 |
| | Fig. 23 Schematic diagram of different chromophore systems, which were built by loading of extracted biphenyl-bridged PMO with different dye molecules. The red arrows show the route of the energy transfer from donors to acceptors, reproduced from ref. 233 with permission from Wiley. | |
Table 2 Summary of the synthesized materials with the energy transfer efficiency from chromophore 1 to chromophore 2 (E12), from chromophore 2 to chromophore 3 (E23), and of two-step-FRET from chromophore 1 through chromophore 2 to chromophore 3 (E123)a233
| Chr1 |
Chr2 |
Chr3 |
Emission maximab |
E12 (%) |
E23 (%) |
E123 (%) |
| Donors |
Acceptors |
| The wavelength of the emission maxima of acceptors and the values of the energy transfer efficiency are represented for samples with maximal concentration of the acceptors before the onset of emission quenching of the acceptors occurs. Chr = chromophore. Emission maxima of the last chromophore. |
| Biphenyl-PMO |
— |
— |
374 |
— |
— |
— |
| Biphenyl-PMO |
Aminocridone |
— |
519 |
95 |
— |
— |
| Biphenyl-PMO |
Diethylaminocouramin |
— |
477 |
91 |
— |
— |
| Chr1 |
Chr2 |
Chr3 |
Emission maximab |
E12 (%) |
E23 (%) |
E123 (%) |
| Donors |
Mediators |
Acceptors |
| Biphenyl-PMO |
Aminocridone |
Dibenzothiacarbocyanine |
630 |
95 |
84 |
80 |
| Biphenyl-PMO |
Aminocridone |
Indocarbocyanine |
692 |
95 |
78 |
74 |
| Biphenyl-PMO |
Diethylaminocouramin |
Dibenzothiacarbocyanine |
634 |
91 |
80 |
73 |
| Biphenyl-PMO |
Diethylaminocouramin |
Sulforodamine |
567 |
91 |
76 |
69 |
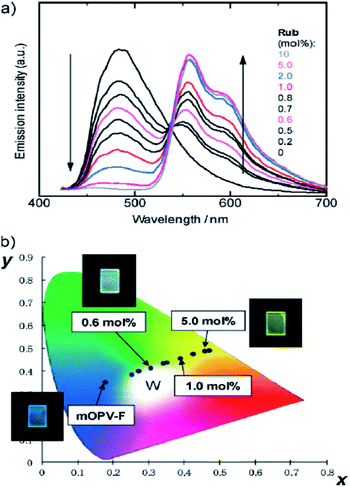 |
| | Fig. 24 (a) Fluorescence spectra of mOPV-F films doped with Rub (0–10 mol% ratio to the OPV unit). (b) Emission colours in the CIE 1931 chromaticity diagram calculated from the fluorescence spectra of Ru-doped mOPV-F films and the photographs of the films under 365 nm UV irradiation, reproduced from ref. 229 with permission from Wiley. | |
Another important study241 has shown that biphenyl-bridged PMO was chosen due to its well-defined mesoporous framework and strong light absorption capacity where viologen functions and Pt were used as the electron acceptor and catalyst, respectively. The purpose of covalently attachment of viologen functions into the PMO framework is not only to create charge transfer (CT) complexes with the biphenyl segments but also to induce electron transfer from biphenyl segments to viologen to promote persistent charge separation.241 It is also reported formation of a 100% benzene-bridged PMO bearing arenetricarbonyl complexes to produce a CT band from Cr to the benzene ring but no photocatalysis was occurred.162 Therefore, Inagaki et al.241 have employed Pt as a catalyst for H2 evolution using Pt/Vio-Bp-PMO in the presence of reduced nicotinamide adenine dinucleotide (NADH) as a sacrificial agent (irradiation at 400 nm). A presumed mechanism was proposed in Fig. 25 for the charge separation.241 In order to confirm this result, the use of homogeneous solution of Bp molecules, methylviologen, and colloidal Pt did not produce any H2, due to a weaker electron-donating feature of molecular Bp than that of densely packed Bp in Bp-PMO. These results clearly indicated the importance of densely packed Bp and Vio within mesoporous structure for hydrogen evolution. Recently, visible-light absorbers acridone (Acd) and methyllacridone (MeAcd) were embedded in the pore walls of PMO to produce well-defined hybrid Acd-PMOs and MeAcd-PMOs.242 The pore sizes of 4.6 and 3.7 nm for Acd-PMO and MeAcd-PMO, respectively, are providing enough space for encapsulation of RuII–ReI complex which has van der Waals radii (ca. 2.4 nm). The encapsulation of RuII and ReI in the PMOs (Acd-PMO or MeAcd-PMO) was worked as photosensitizer and catalyst, respectively for CO2 reduction.242
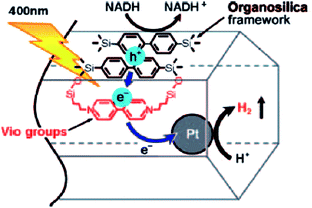 |
| | Fig. 25 Designed solid-state donor–acceptor system based on PMO for photocatalytic H2 evolution. Photo-irradiation induces electron transfer from Bp in the framework to viologen covalently attached to the framework. Pt in the mesochannels promotes H2 evolution. Holes in the framework are consumed by oxidation of the sacrificial agent, reproduced from ref. 241 with permission from Wiley. | |
3.11 Hole-transporting function, optical and solar cell properties
As discussed previously, PMOs with different types of active organic bridging groups (R) ranging from aliphatic and aromatic hydrocarbons to heterocyclics and metal complexes could be used for various target oriented applications. Meanwhile, a number of PMOs with various organic groups including ethane, aromatic and π-conjugated bridging groups have been reported with useful properties such as optical, strong light absorption, fluorescence, light harvesting and excitation energy transfer.20–90,243–248 Recent aromatic organosilica materials have been reported with hole-transporting and optical functions.59 It is anticipated that the emission and charge transport properties of the active organic material could be greatly affected by their confinement within the nanochannels of porous framework which may be well-suited for organic electronic applications such as light-emitting diodes, solar cells, and thin film transistors. Wahab et al.59 have reported the use of evaporation induced self-assembly method (EISA) to organize and chemically bind a photoluminesent/hole transporting material. N,N′-bis(4-tert-butylphenyl)-N,N′-bis(4-((E)-2-(triethoxysilyl)vinyl)phenyl)biphenyl-4,4′-diamine (TEVS-TPD) was incorporated into the ordered nanochannels within an organosilica matrix based on 1,2-bis(triethoxysilyl)ethane (BTSE). This strategy was used to fabricate photoluminescent (PL) and charge transport (CT) groups functionalized nanocomposite thin films in situ wherein the PL/CT material is confined and chemically bonded inside the nanochannels of the PMO. The resultant thin films show interesting photoluminescent and increased hardness properties over their non-ordered counterparts to strongly suggest that the pore channels are occupied by the functionalized material of interest. Later, TEVS-TPD has been used for evaluating the device performance as an improved alternative to poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) PEDOT:PSS as application in organic light emitting diodes (OLEDs) and organic photovoltaics (OPVs) (Fig. 26).243 The devices with cross-linked TEVS-TPD HITL (hole injection/transport layers) (10 nm thickness) exhibited lower turn-on voltage with lower leakage current, higher luminance and stability at high current density, and similar maximum efficiencies at low voltages as compared to those based on PEDOT:PSS HITL. In addition, the devices based on cross-linked TEVS-TPD exhibited device performances superior to those of the devices prepared with TEVS-TPD monomer or TEPCS-TPD polymer that contains insulating moieties between the silicon atom and the aromatic amine. Inagaki et al.248 have reported the synthesis of a hole-transporting periodic mesostructured organosilica (Fig. 27). The hole-transporting behavior of the mesostructured film was examined by TOF measurements. The hole mobility in mesostructured film was ∼3.2 × 10−5 cm2 V−1 s−1 that is slightly lower than that of the hole mobility (∼5.1 × 10−5 cm2 V−1 s−1) determined by non-structured organosilica film. This study suggested that molecular packing of uncondensed precursor in the crystal lattice may also impede hole hopping between organic moieties.
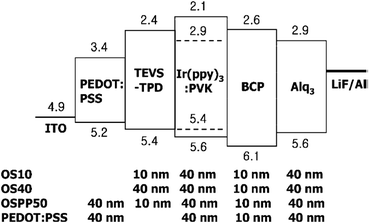 |
| | Fig. 26 Device structures [ITO/HITL (OS10, OS40, OSPP50, PEDOT:PSS)/Ir(ppy)3:PVKemissive layer (40 nm)/BCP (10 nm)/Alq3 (40 nm)/LiF (1 nm)/Al (100 nm)] and the corresponding energy level diagram. The energy levels of the materials except cross-linked TEVS-TPD are literature values, reproduced from ref. 243 with permission from ACS Publications. | |
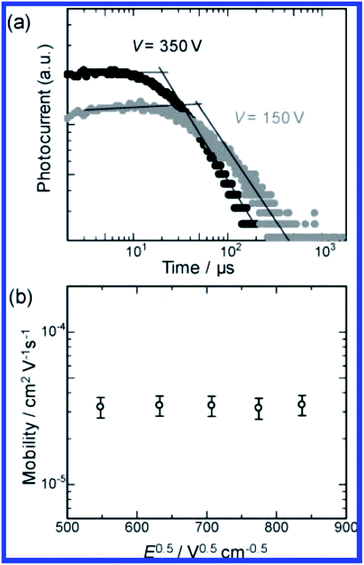 |
| | Fig. 27 (a) Double logarithmic plots of transient photocurrent of 1-Brij76 F (thickness: 5 μm) as a function of time at applied voltages of 150 and 350 V. (b) Plot of the hole mobility of 1-Brij76 F versus electric field strength, reproduced from ref. 248 with permission from ACS Publications. | |
3.12 PMOs with electroactive functions and fuel cell application
The incorporation of electro active functions into the frameworks of PMOs has really shown much interest in developing redox catalysts, electrochemical sensor, electroluminescent systems and electronic devices.20–26 For example, ethane-PMOs with arene-sulfonic acid groups have displayed an acid capacity and proton conductivity of 1.38 meq g−1 and 1.6 × 10−2 S cm−1 respectively.249 PMOs functionalized with electroactive bridged silsesquioxane precursor, N,N′-bis(4′-(3-triethoxysilylpropylureido) phenyl)-1,4-quinonene-diimine (TSUPQD) has shown similar electrochemical behavior as polyaniline but with slightly higher redox peak potentials which are attributed to the electron withdrawing ureidopropyl groups attached to the main chain of the amino-capped aniline trimer.250 One well defined redox pair of peaks was found at around 0.55 V against the reference electrode (Ag/AgCl) which corresponds to the transition from the “leucoemeraldine” to the “emeraldine” form of the aniline oligomer and represents the removal/addition of two electrons. The second redox pair of peaks which corresponds to the oxidation of the “emeraldine” form to the “pernigraniline” form cannot be detected in aqueous solution due to the background range limit from water. In addition, the conductivity measurement was conducted (10.5 S cm−1) for HCl-doped 66 wt% TSU-PMO using a four-probe method. In contrast to the aniline trimer, this measurement shows a two orders of magnitude decrease in conductivity, which arises from the presence of the non-conducting inorganic silica framework.
3.13 Low dielectric layers for microelectronics application
An ultra-low-k layer semiconductor is a material with a dielectric constant (k) much smaller than that of the silicon dioxide used in chips (aprox. 4). The development of novel synthetic strategies is necessary for advancing technologies in the semiconductor industry. Ultralow k layers (k < 2.0) are required for designing modern ICs and other digital devices. It is found to be difficult to make such devices with compact and dense materials with low-k less than 2. In this context, PMOs can play important role because of high amount of hydrophobic organic segments in the framework, which could easily be polarised only to a lesser extent than SiO2.251–258 Ozin et al.252 have reported spin-coated 1,3,5-tris[diethoxysila]cyclohexane [(EtO)2SiCH2]3 cyclic silsesquioxane based PMO thin films with low k values about 2. Interestingly, the dielectric k values of PMO thin films are found to decrease linearly with increasing loading of organic functional groups. Same group has reported PMO thin film of octavinyl polyhedral oligomeric silsesquioxane (OVPOSS) octa(triethoxysilylethyl)POSS(POSS[C2H4Si(OEt)3]8).255 The final PMO film has decreased dielectric constant (k) from 2.03 (pure POSS film) to 1.73 (OV-POSS-PMO film) due to the 10-fold increase in the porosity of the POSS-PMO. Recently, Driesscche et al.258 have reported a new procedure to seal the pores of mesoporous low-k films using a pre-condensed organosilica oligomers pore sealing method for the preparation of low-k mesoporous ethylene-bridged organosilica films.257 The k-value of the non-sealed ethylene-bridged organosilica film was 2.15 for a porosity of 39.5% whereas after the sealing the porosity decreases to 38% and the k-value only slightly increases to 2.22. Even more, no water adsorption was observed on the sealed films. These results show that the sealing almost has no impact on the overall k-value and thus a huge step forward is achieved in sealing mesoporous low-k materials. In addition, ultra-low-k cyclic carbon-bridged PMO films have not only a high chemical resistance but also are hydrophobic and crack-free highly porous films with low-k value of 1.8.258 Fig. 28 suggested that the dielectric constant is stable with frequency as it is expected for hydrophobic low-k films in the explored frequency range.258 The main text of the article should appear here with headings as appropriate.
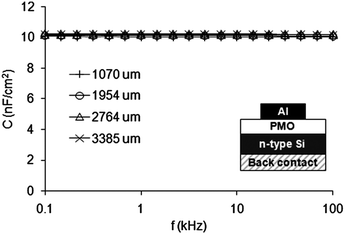 |
| | Fig. 28 Measured accumulation capacitance per unit area as a function of frequency for the four dots of different diameters, reproduced from ref. 258 with permission from the Royal Society of Chemistry. | |
4. Conclusions
This review has clearly demonstrated the recent advances of PMOs with various organic functionalities in the framework for functional group based applications since they were firstly synthesized in 1999. It is found that the intelligent design and synthesis of PMO structures along with new functional groups can provide a completely new application portfolio. For example, studies of PMOs with novel organic functional groups such as chromophores, fluorophores, light-harvesting group, electroactive species and metal complexes have clearly shown advances in the fields of luminescence, opto-electronic devices, solid-state catalysts, and solid state photoreaction systems. For clearer example, a photocatalytic system for CO2 reduction exhibiting visible light harvesting was developed by preparing a hybrid consisting of a supramolecular metal complex as photocatalyst and PMO as light harvester, indicating the influence of chemical structure of organic functional groups in the framework. Moreover, the use of PMOs in biomedical science could be considered as a remarkable advance. Very recently, an efficient and versatile chemical homology principle to synthesize a new family of third-generation PMO nanoparticles with multiple organic groups incorporated within the organosilica framework known as hybridized hollow PMO nanoparticles has been reported for biomedicine.157 In particular, the integration of physiologically active thioethergroups with disulfide bonds has enabled unique reducing and HIFU-responsive drug-releasing performance, specific biological effects, and enhanced ultrasonography behavior for HMONs-based drug delivery systems. Importantly, the Dox loaded PMO nanoparticles exhibited enhanced antitumor therapeutic efficiency both in vitro and in vivo. The MPMO NPs (known as mixed PMO nanoparticles) were found to be very efficient for anti-cancer drug delivery combined with two-photon therapy in MCF-7 breast cancer cells, leading down to 76% cancer cell death. MPMO NPs are thus very promising for nanomedicine applications. These PMOs provide great possibilities for designing suitable host-matrices for targeted application through the careful design of suitable bridges that are able to interact with functional groups of the tested drugs. These studies on PMOs suggest the control ability of the drug loading and their subsequent release. The good biocompatibility and low cytotoxicity of PMOs open challenging opportunities for the use of PMOs as biomaterials in the future. However, some aspects related to pore orientation for biomedical and other devices have to be carefully studied and remain still a research challenge. For example, PMOs with perpendicular pore channels to surface/any substrate have not been studied since electron transfer and motion of biological molecules related to electron transfer are found to depend on the pore orientation. More importantly when any molecules are confined by pore can significantly impact on the final properties including kinetics and releasing profile and so many other properties.
In another field, PMOs synthesized with different morphologies can be directly converted into unique porous carbon structures (pore channels could be changed) depending on the starting reacting materials/precursors. This approach could be extended as a general strategy to prepare ordered mesoporous carbon structures with diverse compositions and morphologies simply by replacing silica from the framework. Similarly, PMOs with nitrogen functions under desired conditions can be converted into carbon nitride and related materials.
List of acronyms used in this paper
| BTSE | 1,2-Bis(triethoxysilyl)ethane |
| 3-APTMOS | 3-Aminopropyltrimethoxysilane |
| BCP | 2,9-Dimethyl-4,7-diphenyl-1,10-phenanthroline |
| AS-MES | Arene sulfonic acid ethane-silica |
| [PhM(CO)3] (M = Cr, Mo) | Arenetricarbonyl complexes |
| Acd-PMO | Acridone functionalized PMO |
| BTEEE | Bis-(triethoxysilyl)ethylene |
| BTEB | Bis(triethoxysilyl)phenylene |
| BTEE | Bis-(triethoxysilyl)ethane |
| BTEBP | Bis(triethoxysilyl)-biphenyl |
| BINAP | Bis-triethoxysilyl-binapthyl |
| CT | Charge transport |
| CLSM | Confocal laser scanning microscopy |
| Dox | Doxorubicin |
| (EtO)3Si–R–Si(OEt)3 | R for ethane, methane, benzene, biphenyl, whereas Eto is for ethoxy group |
| EBPEDS | Ethane-bis(propyl)ethylenediamine silica |
| EISA | Evaporation-induced self-assembly |
| GSH | Glutathione |
| HPLC | High performance liquid chromatography |
| HITL | Hole injection/transport layers |
| HPMO | Hollow morphology of PMO |
| HRP | Horseradish peroxidase |
| NMOF | Light-harvesting metal–organic frameworks |
| MRI | Magnetic resonance imaging |
| MCM | Mobil composition of matter |
| DMPy-PMOs | N,N′-Disilylated pyridine-bridged periodic mesoporous organosilicas |
| TSUPQD | N,N′-Bis(4′-(3-triethoxysilylpropylureido) phenyl)-1,4-quinonene-diimine |
| BTSPED | N,N′-Bis[3-(trimethoxysilyl)propyl]-ethylenediamine |
| TEVS-TPD | N,N′-Bis(4-tert-butylphenyl)-N,N′-bis(4-((E)-2-(triethoxysilyl)vinyl)phenyl)biphenyl-4,4′-diamine |
| OLEDs | Organic light emitting diodes |
| OPV | Organic photovoltaic |
| PRh | Pentyl-linked bis(rhodamine) |
| PMO | Periodic mesoporous organosilica |
| PBS | Phosphate buffer solution |
| PL | Photoluminescent |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PSS | Poly(styrenesulfonate) |
| PMS | Pure mesoporous silica |
| RPLC | Reversed phase liquid chromatography |
| Re | Rhenium |
| SBF | Simulated body fluid |
| TC | Tetracycline |
| TEOS | Tetraethyl orthosilicate |
| Alq3 | Tris(8-hydroxyquinolinato)aluminum |
Acknowledgements
Authors acknowledge the support received for this manuscript from Nanomaterials Centre of Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, Australia.
Notes and references
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Lson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- A. Kuperman, S. Nadimi, S. Oliver, G. A. Ozin, J. M. Garces and M. M. Olken, Nature, 1993, 365, 239–242 CrossRef CAS PubMed.
- S. Inagaki, Y. Fukushima and K. Kuroda, J. Chem. Soc., Chem. Commun., 1993, 680–683 RSC.
- S. L. Burkett, S. D. Sims and S. Mann, Chem. Commun., 1996, 1367–1368 RSC.
- D. J. Macquarrie, Chem. Commun., 1996, 1961–1962 RSC.
- G. Cerveau, R. J. P. Corriu, E. Framery and F. Lerouge, Chem. Mater., 2004, 16, 3794–3799 CrossRef CAS.
- D. A. Loy, G. M. Jamison, B. M. Baugher, S. A. Myers, R. A. Assink and K. J. Shea, Chem. Mater., 1996, 8, 656–663 CrossRef CAS.
- B. Boury, R. J. P. Corriu and V. L. Strat, Chem. Mater., 1999, 11, 2796–2803 CrossRef CAS.
- D. A. Loy and K. J. Shea, Chem. Rev., 1995, 95, 1431–1442 CrossRef CAS.
- G. Cerveau, S. Chappellet, R. J. P. Corriu, B. Dabiens and J. L. Bideau, Organometallics, 2002, 21, 1560–1564 CrossRef CAS.
- D. A. Loy, B. M. Baugher, C. R. Baugher, D. A. Schneider and K. Rahimian, Chem. Mater., 2000, 12, 3624–3632 CrossRef CAS.
- H. Skar, J. G. Seland, Y. Liang, N. A. Frøystein, K. W. Törnroos and R. Anwander, Eur. J. Inorg. Chem., 2013, 5969–5979 CrossRef CAS PubMed.
- V. Morales, J. A. Villajos and R. A. Garcıa, J. Mater. Sci., 2013, 48, 5990–6000 CrossRef CAS.
- Y. Liang and R. Anwander, Dalton Trans., 2013, 42, 12521–12545 RSC.
- A. Corma, Chem. Rev., 1997, 97, 2373–2420 CrossRef CAS PubMed.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS.
- M. H. Lim, C. F. Blanford and A. Stein, J. Am. Chem. Soc., 1997, 119, 4090–4091 CrossRef CAS.
- C. H. Zhou, X. Xia, C. X. Lin, D. S. Tonga and J. N. Beltramini, Chem. Soc. Rev., 2011, 40, 5588–5617 RSC.
- J. Y. Ying, C. P. Mehner and M. S. Wong, Angew. Chem., Int. Ed., 1999, 38, 56–77 CrossRef CAS.
- B. Hatton, K. Landskron, W. Whitnall, D. Perovic and G. A. Ozin, Acc. Chem. Res., 2005, 38, 305–312 CrossRef CAS PubMed.
- S. S. Park, M. S. Moorthy and C. S. Ha, NPG Asia Mater., 2014, 6, e96, DOI:10.1038/am.2014.13.
- N. Mizoshita, T. Taniab and S. Inagaki, Chem. Soc. Rev., 2011, 40, 789–800 RSC.
- C. H. Zhou, J. N. Beltramini, Y. X. Fan and M. Lu, Chem. Soc. Rev., 2008, 37, 527–549 RSC.
- F. Hoffmann, M. Cornelius, J. Morell and M. Froba, Angew. Chem., Int. Ed., 2006, 45, 3216–3251 CrossRef CAS PubMed.
- M. Jaroniec, Nature, 2006, 442, 638–640 CrossRef CAS PubMed.
- W. Wang, J. E. Lofgreen and G. A. Ozin, Small, 2010, 6, 2634–2642 CrossRef CAS PubMed.
- P. V. D. Voort, D. Esquivel, E. D. Canck, F. Goethals, I. V. Driessche and F. J. Romero-Salguero, Chem. Soc. Rev., 2013, 42, 3913–3955 RSC.
- F. Hoffmann and M. Froba, Chem. Soc. Rev., 2011, 40, 608–620 RSC.
- S. Inagaki, S. Guan, T. Ohsuna and O. Terasaki, Nature, 2002, 416, 304–307 CrossRef CAS PubMed.
- M. P. Kapoor and S. Inagaki, Bull. Chem. Soc. Jpn., 2006, 79, 1463–1475 CrossRef CAS.
- W. J. Hunks and G. A. Ozin, J. Mater. Chem., 2005, 15, 3716–3724 RSC.
- A. Stein, B. J. Melde and R. C. Schroden, Adv. Mater., 2000, 12, 1403–1419 CrossRef CAS.
- A. Sayari and S. Hamoudi, Chem. Mater., 2001, 13, 3151–3168 CrossRef CAS.
- A. Sayari and P. Liu, Microporous Mesoporous Mater., 1997, 12, 149–177 CrossRef CAS.
- A. Bhaumik and T. Tatsumi, J. Catal., 2000, 189, 31–39 CrossRef CAS.
- F. Babonneau, L. Leite and S. Fontlupt, J. Mater. Chem., 1999, 9, 175–178 RSC.
- A. Corma, J. L. Jorda, M. T. Navarro and F. Rey, Chem. Commun., 1998, 1899–1900 RSC.
- W. M. V. Rhijin, D. E. D. Vos, B. F. Sels, W. D. Bossaert and P. A. Jacobs, Chem. Commun., 1998, 317–318 RSC.
- J. Liu, Y. Shin, Z. Nie, J. H. Chang, L. Q. Wang, G. E. Fryxell, W. D. Samuels and G. J. Exarhos, J. Phys. Chem. A, 2000, 104, 8328–8339 CrossRef CAS.
- S. Inagaki, S. Guan, Y. Fukushima, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 1999, 121, 9611–9614 CrossRef CAS.
- B. J. Melde, B. T. Holland, C. F. Blanford and A. Stein, Chem. Mater., 1999, 11, 3302–3308 CrossRef CAS.
- T. Asefa, M. J. MacLachlan, N. Coombs and G. A. Ozin, Nature, 1999, 402, 867–871 CAS.
- C. Yoshina-Ishii, T. Asefa, N. Coombs, M. J. MacLachlan and G. A. Ozin, Chem. Commun., 1999, 2539–2540 RSC.
- S. Guan, S. Inagaki, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 2000, 122, 5660–5661 CrossRef CAS.
- A. Sayari, S. Hamoudi, Y. Yang, I. L. Moudrakovski and J. R. Ripmeester, Chem. Mater., 2000, 12, 3857–3863 CrossRef CAS.
- D. Zhang, J. Xu, Q. Zhao, T. Cheng and G. Liu, ChemCatChem, 2014, 6, 2998–3003 CrossRef CAS PubMed.
- M. P. Kapoor and S. Inagaki, Chem. Mater., 2002, 14, 3509–3514 CrossRef CAS.
- M. Kruk, M. Jaroniec, S. Guan and S. Inagaki, J. Phys. Chem. B, 2001, 105, 681–689 CrossRef CAS.
- T. Asefa, M. J. MacLachlan, H. Grondey, N. Coombs and G. A. Ozin, Angew. Chem., Int. Ed., 2000, 39, 1808–1811 CrossRef CAS.
- S. Hamoudi, Y. Yang, I. L. Moudrskovski, S. Lang and A. Sayari, J. Phys. Chem. B, 2001, 105, 9118–9123 CrossRef CAS.
- H. G. Zhu, D. J. Jones, J. Zajac, J. Roziere and R. Dutartre, Chem. Commun., 2001, 2568–2569 RSC.
- H. I. Lee, C. Pak, S. H. Yi, J. K. Shon, S. S. Kim, B. G. So, H. Chang, J. E. Yie, Y. U. Kwon and J. M. Kim, J. Mater. Chem., 2005, 15, 4711–4717 RSC.
- M. A. Wahab, I. Kim and C. S. Ha, J. Solid State Chem., 2004, 177, 3439–3447 CrossRef CAS PubMed.
- M. A. Wahab, H. Q. Eliza, X. H. Lu and C. B. He, Soft Mater., 2010, 8, 183–196 CrossRef PubMed.
- M. A. Wahab and C. B. He, Soft Mater., 2009, 7, 79–92 CrossRef PubMed.
- M. A. Wahab, H. Hussain and C. B. He, Langmuir, 2009, 25, 4743–4750 CrossRef CAS PubMed.
- M. A. Wahab and C. B. He, Langmuir, 2009, 25, 832–838 CrossRef CAS PubMed.
- M. A. Wahab and C. B. He, J. Nanosci. Nanotechnol., 2008, 8, 6381–6386 CrossRef PubMed.
- M. A. Wahab, S. Sudhakar, Y. Elaine and A. Sellinger, Chem. Mater., 2008, 20, 1855–1861 CrossRef CAS.
- M. A. Wahab, I. Kim and C. S. Ha, J. Nanosci. Nanotechnol., 2008, 8, 3532–3538 CrossRef PubMed.
- M. A. Wahab and A. Sellinger, Chem. Lett., 2006, 35, 1240–1241 CrossRef.
- M. A. Wahab, I. Imae, Y. Kawakami and C. S. Ha, Microporous Mesoporous Mater., 2006, 92, 201–211 CrossRef CAS PubMed.
- M. A. Wahab, I. Imae, Y. Kawakami and C. S. Ha, Chem. Mater., 2005, 17, 2165–2174 CrossRef CAS.
- M. A. Wahab and C. S. Ha, J. Mater. Chem., 2005, 15, 508–516 RSC.
- M. A. Wahab, I. Kim and C. S. Ha, J. Solid State Chem., 2004, 177, 3439–3447 CrossRef CAS PubMed.
- M. A. Wahab, I. Kim and C. S. Ha, Microporous Mesoporous Mater., 2004, 69, 19–27 CrossRef CAS PubMed.
- M. A. Wahab, S. S. Chu, C. Anand and C. S. Ha, J. Nanosci. Nanotechnol., 2012, 12, 4531–4539 CrossRef PubMed.
- S. Hamoudi and S. Kaliaguine, Chem. Commun., 2002, 2118–2119 RSC.
- O. Muth, C. Schellbach and M. Froba, Chem. Commun., 2001, 2032–2033 RSC.
- M. C. Burleigh, M. A. Markowitz, M. S. Spector and P. B. Gaber, J. Phys. Chem. B, 2001, 105, 9935–9942 CrossRef CAS.
- J. R. Matos, M. Kurk, L. P. Mercuri, M. Jeroniec, T. Asefa, N. Coombs, G. A. Ozin, T. Kamiyama and O. Terasaki, Chem. Mater., 2002, 14, 1903–1905 CrossRef CAS.
- C. Yu, Y. Yu and D. Zhao, Chem. Commun., 2000, 575–576 RSC.
- W. Guo, J. Y. Park, M. O. Oh, H. W. Jeong, W. J. Cho, I. Kim and C. S. Ha, Chem. Mater., 2003, 15, 2295–2298 CrossRef CAS.
- W. Guo, I. Kim and C. S. Ha, Chem. Commun., 2003, 2692–2693 RSC.
- K. Nakajima, D. Lu, I. Tomita, S. Inagaki, M. Hara, S. Hayashi, K. Domen and J. N. Kondo, Chem. Lett., 2003, 32, 950–951 CrossRef CAS.
- W. Wang, S. Xie, W. Zhou and A. Sayari, Chem. Mater., 2004, 16, 1756–1762 CrossRef CAS.
- K. Nakajima, I. Tomita, M. Hara, S. Hayashi, K. Domen and J. N. Kondo, J. Mater. Chem., 2005, 15, 2362–2368 RSC.
- M. P. Kapoor, N. Setoyama, Q. Yang, M. Ohashi and S. Inagaki, Langmuir, 2005, 21, 443 CrossRef CAS PubMed.
- Y. Goto and S. Inagaki, Chem. Commun., 2002, 2410–2411 RSC.
- G. Temtsin, T. Asefa, S. Bittner and G. A. Ozin, J. Mater. Chem., 2001, 11, 3202–3206 RSC.
- M. Kuroki, T. Asefa, W. Whitnal, M. Kruk, C. Yoshina-Ishii, M. Jaroniec and G. A. Ozin, J. Am. Chem. Soc., 2002, 124, 13886–13895 CrossRef CAS PubMed.
- K. Landskron, B. D. Hatton, D. D. Perovic and G. A. Ozin, Science, 2003, 302, 266–269 CrossRef CAS PubMed.
- K. Landskron and G. A. Ozin, Science, 2004, 306, 1529–1532 CrossRef CAS PubMed.
- W. J. Hunks and G. A. Ozin, Chem. Commun., 2004, 2426–2427 RSC.
- W. J. Hunks and G. A. Ozin, Chem. Mater., 2004, 16, 5465–5472 CrossRef CAS.
- J. Morell, G. Wolter and M. Froba, Chem. Mater., 2005, 17, 804–808 CrossRef CAS.
- K. Okamoto, Y. Goto and S. Inagaki, J. Mater. Chem., 2005, 15, 4136–4140 RSC.
- N. Bion, P. Ferreira, A. Valente, I. S. Goncalves and J. Rocha, J. Mater. Chem., 2003, 13, 1910–1913 RSC.
- B. Onida, L. Borello, C. Busco, P. Ugliengo, Y. Goto, S. Inagaki and E. Garrone, J. Phys. Chem., 2005, 109, 11961–11966 CrossRef CAS PubMed.
- Y. Goto, K. Okamoto and S. Inagaki, Bull. Chem. Soc. Jpn., 2005, 78, 932–936 CrossRef CAS.
- M. P. Kapoor, Q. Yang and S. Inagaki, Chem. Mater., 2004, 16, 1209–1213 CrossRef CAS.
- M. P. Kapoor, Q. Yang and S. Inagaki, J. Am. Chem. Soc., 2002, 124, 15176–15177 CrossRef CAS PubMed.
- T. Shimada, K. Aoki, Y. Shinoda, T. Nakamura, N. Tokunaga, S. Inagaki and T. Hayashi, J. Am. Chem. Soc., 2003, 125, 4688–4689 CrossRef CAS PubMed.
- M. P. Kapoor, S. Inagaki, S. Ikeda, K. Kakiuchi, M. Suda and T. Shimada, J. Am. Chem. Soc., 2005, 127, 8174–8178 CrossRef CAS PubMed.
- K. Kamoto, M. P. Kapoor and S. Inagaki, Chem. Commun., 2005, 1423–1424 RSC.
- Y. Xia, W. Wang and R. Mokaya, J. Am. Chem. Soc., 2005, 127, 790–798 CrossRef CAS PubMed.
- W. Wang, W. Zhou and A. Sayari, Chem. Mater., 2003, 15, 4886–4889 CrossRef CAS.
- A. Sayari and W. Wang, J. Am. Chem. Soc., 2005, 127, 12194–12195 CrossRef CAS PubMed.
- J. Liu, H. Q. Yang, F. Kleitz, Z. G. Chen, T. Yang, E. Strounina, M. Lu and S. Z. Qiao, Adv. Funct. Mater., 2012, 22, 591–599 CrossRef CAS PubMed.
- S. Z. Qiao, C. X. Lin, Y. G. Jin, Z. Li, Z. Yan, Z. P. Hao, Y. N. Huang and M. Lu, J. Phys. Chem. C, 2009, 113, 8673–8682 CAS.
- C. X. Lin, S. Z. Qiao, C. Z. Yu, S. Ismadji and M. Lu, Microporous Mesoporous Mater., 2009, 117, 213–219 CrossRef CAS PubMed.
- L. Zhang, S. Z. Qiao, Y. G. Jin, Z. G. Chen, H. C. Gu and M. Lu, Adv. Mater., 2008, 20, 805–809 CrossRef CAS PubMed.
- J. Brown, L. Mercier and T. J. Pinnavaia, Chem. Commun., 1999, 69–70 RSC.
- T. Asefa, M. Kruk, M. J. MacLachlan, N. Coombs, H. Grondey, M. Jaroniec and G. A. Ozin, J. Am. Chem. Soc., 2001, 1235, 8520–8530 CrossRef PubMed.
- X. Wu, S. Ji, Y. Li, B. Li, X. Zhu, K. Hanabusa and Y. Yang, J. Am. Chem. Soc., 2009, 131, 5986–5993 CrossRef CAS PubMed.
- S. MacQuarrie, M. P. Thompson, A. Blanc, N. J. Mosey, R. P. Lemieux and C. M. Crudden, J. Am. Chem. Soc., 2008, 130, 14099–14101 CrossRef CAS PubMed.
- X. Wu and C. M. Crudden, Chem. Mater., 2012, 24, 3839–3846 CrossRef CAS.
- G. Zhu, H. Zhong, Q. Yang and C. Li, Microporous Mesoporous Mater., 2008, 116, 36–43 CrossRef CAS PubMed.
- T. Seki, K. McEleney and C. M. Crudden, Chem. Commun., 2012, 48, 6369–6371 RSC.
- A. Kuschel and S. Polarz, J. Am. Chem. Soc., 2010, 132, 6558–6565 CrossRef CAS PubMed.
- J. Morell, S. Chatterjee, P. J. Klar, D. Mauder, I. Shenderovich, H. Hoffmann and M. Froba, Chem.–Eur. J., 2008, 14, 5935–5940 CrossRef CAS PubMed.
- X. Wu, S. Ji, Y. Li, B. Li, X. Zhu, K. Hanabusa and Y. Yang, J. Am. Chem. Soc., 2009, 131, 5986–5993 CrossRef CAS PubMed.
- S. MacQuarrie, M. P. Thompson, A. Blanc, N. J. Mosey, R. P. Lemieux and C. M. Crudden, J. Am. Chem. Soc., 2008, 130, 14099–14101 CrossRef CAS PubMed.
- X. Wu and C. M. Crudden, Chem. Mater., 2012, 24, 3839–3846 CrossRef CAS.
- G. Zhu, H. Zhong, Q. Yang and C. Li, Microporous Mesoporous Mater., 2008, 116, 36–43 CrossRef CAS PubMed.
- T. Seki, K. McEleney and C. M. Crudden, Chem. Commun., 2012, 48, 6369–6371 RSC.
- A. Kuschel and S. Polarz, J. Am. Chem. Soc., 2010, 132, 6558–6565 CrossRef CAS PubMed.
- J. Morell, S. Chatterjee, P. J. Klar, D. Mauder, I. Shenderovich, H. Hoffmann and M. Froba, Chem.–Eur. J., 2008, 14, 5935–5940 CrossRef CAS PubMed.
- Y. Li, S. Wang, M. Xiao, M. Wang, Z. Huang, B. Li and Y. Yang, Nanotechnology, 2013, 24, 035603–035608 CrossRef PubMed.
- X. Meng, T. Yokoi, D. Lu and T. Tatsumi, Angew. Chem., Int. Ed., 2007, 46, 7796–7798 CrossRef CAS PubMed.
- B. Li, Z. Xu, W. Zhuang, Y. Chen, S. Wang, Y. Li, M. Wang and Y. Yang, Chem. Commun., 2011, 47, 11495–11497 RSC.
- V. Jayalakshmi, T. Wood, R. Basu, J. Du, T. Blackburn, C. Rosenblatt, C. M. Crudden and R. P. Lemieux, J. Mater. Chem., 2012, 22, 15255–15261 RSC.
- T. T. Hao, J. Y. Shi, T. Y. Zhuang, W. D. Wang, F. C. Lia and W. Wang, RSC Adv., 2012, 2, 2010–2014 RSC.
- M. W. A. MacLean, T. K. Wood, G. Wu, R. P. Lemieux and C. M. Crudden, Chem. Mater., 2014, 26, 5852–5859 CrossRef CAS.
- J. J. E. Moreau, L. Vellutini, M. W. Chi Man and C. Bied, J. Am. Chem. Soc., 2001, 123, 1509–1510 CrossRef CAS.
- R. A. García-Munnoz, V. Morales, M. Linares and B. Rico-Oller, Langmuir, 2014, 30, 881–890 CrossRef PubMed.
- J. J. E. Moreau, L. Vellutini, M. W. Chi Man and C. Bied, Chem.–Eur. J., 2003, 9, 1594–1599 CrossRef CAS PubMed.
- S. Polarz and A. Kuschel, Adv. Mater., 2006, 18, 1206–1209 CrossRef CAS PubMed.
- M. W. A. MacLean, L. M. Reid, X. Wu and C. M. Crudden, Chem.–Asian J., 2015, 10, 70–82 CrossRef CAS PubMed.
- Y. Jiang, X. Liu, Y. Chen, L. Zhou, Y. He, L. Ma and J. Gao, Bioresour. Technol., 2014, 153, 278–283 CrossRef CAS PubMed.
- B. Karimi, H. M. Mirzaei and A. Mobaraki, Catal. Sci. Technol., 2012, 2, 828–834 CAS.
- Z. Zhou, R. N. K. Taylor, S. Kullmann, H. Bao and M. Hartmann, Adv. Mater., 2011, 23, 2627–2632 CrossRef CAS PubMed.
- S. E. Diez, E. I. Diaz and R. M. A. Blanco, Microporous Mesoporous Mater., 2010, 132, 487–493 CrossRef PubMed.
- N. Li, J. G. Wang, H. J. Zhou, P. C. Sun and T. H. Chen, Chem. Mater., 2011, 23, 4241–4249 CrossRef CAS.
- W. Na, Q. Wei, J. N. Lan, Z. R. Nie, H. Sun and Q. Y. Li, Microporous Mesoporous Mater., 2010, 134, 72–78 CrossRef CAS PubMed.
- S. Shylesh, A. Wagener, A. Seifert, S. Ernst and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 184–187 CrossRef CAS PubMed.
- C. M. Jimenez, J. Croissant, M. Maynadier, X. Cattoen, M. W. C. Man, J. Vergnaud, V. Chaleix, V. Sol, M. Garcia, M. Gary-Bobo, L. Raehma and J. O. Durand, J. Mater. Chem. B, 2015, 3, 3681–3684 RSC.
- M. Beretta, J. Morell, P. Sozzani and M. Froba, Chem. Commun., 2010, 46, 2495–2497 RSC.
- M. Bhattacharyya, P. Hiwale, M. Piras, L. Medda, D. Steri, M. Piludu, A. Salis and M. Monduzzi, J. Phys. Chem. C, 2010, 114, 19928–19934 CAS.
- J. Croissant, D. Salles, M. Maynadier, O. Mongin, V. Hugues, M. B. Desce, X. Cattoën, M. W. C. Man, A. Gallud, M. Garcia, M. G. Bobo, L. Raehm and J. O. Durand, Chem. Mater., 2014, 26, 7214–7220 CrossRef CAS.
- Z. Zhou, A. Inayat, W. Schwieger and M. Hartmann, Microporous Mesoporous Mater., 2012, 154, 133–141 CrossRef CAS PubMed.
- J. Gan, Z. Zhu, G. Yan, Y. Liu, P. Yang and B. Liu, Anal. Chem., 2012, 84, 5809–5815 CrossRef CAS PubMed.
- K. Qian, W. Gu, P. Yuan, F. Liu, Y. Wang, M. Monteiro and C. Yu, Small, 2012, 8, 231–236 CrossRef CAS PubMed.
- Y. Zhou, M. M. Wan, L. Gao, N. Lin, W. G. Lin and J. H. Zhu, J. Mater. Chem. B, 2013, 1, 1738–1748 RSC.
- B. Nohair, P. T. H. Thao, V. T. H. Nguyen, P. Q. Tien, D. T. Phuong, L. G. Hy and S. Kaliaguine, J. Phys. Chem. C, 2012, 116, 10904–10912 CAS.
- N. Wei, W. Qi and Z. Nieuoren, J. Mater. Chem., 2012, 22, 9970–9974 RSC.
- C. X. Lin, Z. Li, S. Brumbley, L. Petrasovits, R. McQualter, C. Yu and M. Lu, J. Mater. Chem., 2011, 21, 7565–7571 RSC.
- M. Park, S. S. Park, S. Selvaraj, I. Kim and C. S. Ha, J. Porous Mater., 2011, 18, 217–223 CrossRef CAS.
- M. S. Moorthy, M. J. Kim, J. H. Bae, S. S. Park, N. Saravanan, S. H. Kim and C. S. Ha, Eur. J. Inorg. Chem., 2013, 3028–3038 CrossRef CAS PubMed.
- C. X. Lin, S. Z. Qiao, C. Z. Yu, S. Ismadji and M. Lu, Microporous Mesoporous Mater., 2009, 117, 213–219 CrossRef CAS PubMed.
- M. Rat, M. H. Zahedi-Niaki, S. Kaliaguine and T. O. Do, Microporous Mesoporous Mater., 2008, 112, 26–31 CrossRef CAS PubMed.
- C. Li, J. Liu, L. Zhang, J. Yang and Q. Yang, Microporous Mesoporous Mater., 2008, 113, 333–342 CrossRef CAS PubMed.
- F. X. Zhu, W. Wang and H. X. Li, J. Am. Chem. Soc., 2011, 133, 11632–11640 CrossRef CAS PubMed.
- E. Y. Jeong, A. Burri, S. Y. Lee and S. E. Park, J. Mater. Chem., 2010, 20, 10869–10875 RSC.
- B. Karimi and F. K. Esfahani, Chem. Commun., 2011, 47, 10452–10454 RSC.
- B. Karimi, D. Elhamifar, J. H. Clark and A. J. Hunt, Org. Biomol. Chem., 2011, 9, 7420–7426 CAS.
- Y. Chen, Q. S. Meng, M. Wu, S. Wang, P. Xu, H. Chen, Y. Li, L. Zhang, L. Z. Wang and J. Shi, J. Am. Chem. Soc., 2014, 136, 16326–16334 CrossRef CAS PubMed.
- D. Esquivel, E. D. Canck, C. J. Sanchidri, P. V. D. Voort and F. J. R. Salguero, J. Mater. Chem., 2011, 21, 10990–10998 RSC.
- M. Ohashi, M. P. Kapoor and S. Inagaki, Chem. Commun., 2008, 841–843 RSC.
- M. P. Kapoor, Y. Kasama, T. Yokoyama, M. Yanagi, S. Inagaki, N. Hironobu and L. R. Juneja, J. Mater. Chem., 2006, 16, 4714–4722 RSC.
- S. E. Hankari, B. Motos-Perez, P. Hesemann, A. Bouhaouss and J. J. E. Moreau, J. Mater. Chem., 2011, 21, 6948–6955 RSC.
- T. Kamegawa, T. Sakai, M. Matsuoka and M. Anpo, J. Am. Chem. Soc., 2005, 127, 16784–16785 CrossRef CAS PubMed.
- C. Baleizao, B. Gigante, D. Das, M. Alvaro, H. Garcia and A. Corma, J. Catal., 2004, 223, 106–113 CrossRef CAS PubMed.
- A. Corma, D. Das, H. Garcia and A. Leyva, J. Catal., 2005, 229, 322–331 CrossRef CAS PubMed.
- F. Zhang, C. M. Kang, Y. Y. Wei and H. X. Li, Adv. Funct. Mater., 2011, 21, 3189–3197 CrossRef CAS PubMed.
- J. L. Huang, F. X. Zhu, W. H. He, F. Zhang, W. Wang and H. X. Li, J. Am. Chem. Soc., 2010, 132, 1492–1493 CrossRef CAS PubMed.
- C. M. Kang, J. L. Huang, W. H. He and F. Zhang, J. Organomet. Chem., 2010, 695, 120–127 CrossRef CAS PubMed.
- E. Y. Jeong, M. B. Ansari and S. E. Park, ACS Catal., 2011, 1, 855–863 CrossRef CAS.
- V. Dufaud, F. Beauchesne and L. Bonneviot, Angew. Chem., Int. Ed., 2005, 44, 3475–3477 CrossRef CAS PubMed.
- X. S. Yang, F. X. Zhu, J. L. Huang, F. Zhang and H. X. Li, Chem. Mater., 2009, 21, 4925–4933 CrossRef CAS.
- Y. Yang, Y. Zhang, S. J. Hao and Q. B. Kan, J. Colloid Interface Sci., 2011, 362, 157–163 CrossRef CAS PubMed.
- X. Wang, D. Lu, R. Austin, A. Agarwal, L. J. Mueller, Z. Liu, J. Wu and P. Feng, Langmuir, 2007, 23, 5735–5739 CrossRef CAS PubMed.
- D. I. Fried, F. J. Brieler and M. Froba, ChemCatChem, 2013, 5, 862–884 CrossRef CAS PubMed.
- J. Gan, J. Zhu, G. Yan, Y. Liu, P. Yang and B. Liu, Anal. Chem., 2012, 84, 5809–5815 CrossRef CAS PubMed.
- Y. M. Huh, Y. W. Jun, H. T. Song, S. Kim, J. S. Choi, J. H. Lee, S. Yoon, K. S. Kim, J. S. Shin, J. S. Suh and J. Cheon, J. Am. Chem. Soc., 2005, 127, 12387–12391 CrossRef CAS PubMed.
- S. Giri, B. G. Trewyn, M. P. Stellmarker and V. S. Y. Lin, Angew. Chem., Int. Ed., 2005, 44, 5038–5044 CrossRef CAS PubMed.
- J. Kim, J. E. Lee, J. Lee, Y. Jang, S. W. Kim, K. An, J. H. Yu and T. Hyeon, Angew. Chem., Int. Ed., 2006, 118, 4907–4911 CrossRef PubMed.
- T. Sen, A. Sebastianelli and I. J. Bruce, J. Am. Chem. Soc., 2006, 128, 7130–7131 CrossRef CAS PubMed.
- M. Arruebo, M. Galan, N. Navascues, C. Tellez, C. Marquina, M. R. Ibarra and J. Santamaria, Chem. Mater., 2006, 18, 1911–1919 CrossRef CAS.
- C. Y. Lai, B. G. Trewyn, D. M. Jeftinija, K. Jeftinija, S. Xu, S. Jeftinija and V. S. Y. Lin, J. Am. Chem. Soc., 2003, 125, 4451–4459 CrossRef CAS PubMed.
- A. H. Lu, W. C. Li, A. Kiefer, W. Schmidt, E. Bill, G. Fink and F. Schuth, J. Am. Chem. Soc., 2004, 126, 8616–8617 CrossRef CAS PubMed.
- L. Zhang, S. Z. Qiao, Y. Jin, Z. Chen, H. Gu and M. Lu, Adv. Mater., 2008, 20, 805–809 CrossRef CAS PubMed.
- S. Biju, Y. Eom, J. C. G. Bunzli and H. K. Kim, J. Mater. Chem. C, 2013, 1, 3454–3466 RSC.
- W. Stoeber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef CAS.
- M. Ide, E. D. Canck, I. V. Driessche, F. Lynenc and P. V. D. Voorta, RSC Adv., 2015, 5, 5546–5552 RSC.
- T. Salesch, S. Bachmann, S. Brugger, R. Rabelo-Schaefer, K. Albert, S. Steinbrecher, E. Plies, A. Mehdi, C. Rey, R. J. P. Corriu and E. Lindner, Adv. Funct. Mater., 2002, 12, 132–142 CrossRef.
- V. Rebbin, R. Schmidt and M. Froba, Angew. Chem., Int. Ed., 2006, 45, 5210–5214 CrossRef CAS PubMed.
- L. Huang, J. Lu, B. Di, F. Feng, M. Su and F. Yan, J. Sep. Sci., 2011, 34, 2523–2527 CrossRef CAS PubMed.
- G. R. Zhu, Q. H. Yang, D. M. Jiang, J. Yang, L. Zhang, Y. Li and C. Li, J. Chromatogr. A, 2006, 1103, 257–264 CrossRef CAS PubMed.
- Y. P. Zhang, Y. Jin, P. Dai, H. Yu, D. H. Yu, Y. X. Ke and X. M. Liang, Anal. Methods, 2009, 1, 123–127 RSC.
- Y. P. Zhang, Y. Jin, P. Dai, H. Yu, D. H. Yu, Y. X. Ke and X. M. Liang, Talanta, 2010, 81, 824–830 CrossRef CAS PubMed.
- G. R. Zhu, D. M. Jiang, Q. H. Yang, J. Yang and C. Li, J. Chromatogr. A, 2007, 1149, 219–227 CrossRef CAS PubMed.
- G. R. Zhu, H. Zhong, Q. Yang and C. Li, Microporous Mesoporous Mater., 2008, 116, 36–43 CrossRef CAS PubMed.
- C. Li, B. Di, W. Q. Hao, F. Yan and M. X. Su, J. Chromatogr. A, 2011, 1218, 408–415 CrossRef CAS PubMed.
- J. Lu, L. L. Huang, B. Di, F. Feng and M. X. Su, Chromatographia, 2011, 74, 515–521 CAS.
- A. Walcarius and L. Mercier, J. Mater. Chem., 2010, 20, 4478–4511 RSC.
- H. Zhu, D. J. Jones, J. Zajac, R. Dutartre, M. Rhomari and J. Roziere, Chem. Mater., 2002, 14, 4886–4894 CrossRef CAS.
- M. Waki, N. Mizoshita, T. Ohsuna, T. Taniab and S. Inagaki, Chem. Commun., 2010, 46, 8163–8165 RSC.
- O. Olkhovyk and M. Jaroniec, J. Am. Chem. Soc., 2005, 127, 60–61 CrossRef CAS PubMed.
- M. C. Burleigh, S. Dai, E. W. Hagaman and J. S. Lin, Chem. Mater., 2001, 13, 2537–2546 CrossRef CAS.
- P. J. Chiu, S. Vetrivel, A. S. T. Chiang and H. M. Kao, New J. Chem., 2011, 35, 489–494 RSC.
- S. A. Trammell, B. J. Melde, D. Zabetakis, J. R. Deschamps, M. A. Dinderman, B. J. Johnson and A. W. Kusterbeck, Sens. Actuators, B, 2011, 155, 737–744 CrossRef CAS PubMed.
- S. A. Trammell, M. Zeinali, B. J. Melde, P. T. Charles, F. L. Velez, M. A. Dinderman, A. Kusterbeck and M. A. Markowitz, Anal. Chem., 2008, 80, 4627–4633 CrossRef CAS PubMed.
- M. C. Burleigh, M. A. Markowitz, M. S. Spector and B. P. Gaber, Environ. Sci. Technol., 2002, 36, 2515–2518 CrossRef CAS.
- S. Jayasundera, M. C. Burleigh, M. Zeinali, M. S. Spector, J. B. Miller, W. F. Yan, S. Dai and M. A. Markowitz, J. Phys. Chem. B, 2005, 109, 9198–9201 CrossRef CAS PubMed.
- J. Peng, Y. Yao, X. Zhang, C. Li and Q. Yang, Chem. Commun., 2014, 50, 10830–10833 RSC.
- J. Du, J. C. Wechsler, J. M. Lobez, H. P. Loock and C. M. Crudden, Small, 2010, 6, 1168–1172 CrossRef CAS PubMed.
- B. J. Melde and B. J. Johnson, Anal. Bioanal. Chem., 2010, 398, 1565–1573 CrossRef CAS PubMed.
- S. S. Park, B. An and C. S. Ha, Microporous Mesoporous Mater., 2008, 111, 367–378 CrossRef CAS PubMed.
- B. J. White, M. Zeinali, K. M. Shafer, C. H. Patterson Jr, P. T. Charles and M. A. Markowitz, Biosens. Bioelectron., 2007, 22, 1154–1162 CrossRef PubMed.
- J. Lin, Z. Wei and C. Mao, Biosens. Bioelectron., 2011, 29, 40–45 CrossRef CAS PubMed.
- X. Qiu, S. Han, Y. Hu and B. Sun, Chem.–Eur. J., 2015, 21, 4126–4132 CrossRef CAS PubMed.
- E. D. Canck, C. Vercaemst, F. Verpoort and P. V. D. Voort, Stud. Surf. Sci. Catal., 2010, 175, 365–368 CrossRef.
- B. J. Johnson, N. E. Anderson, P. T. Charles, A. P. Malanoski, B. J. Malde, M. Nasir and J. R. Deschamps, Sensors, 2011, 11, 886–904 CrossRef CAS PubMed.
- B. J. Johnson, B. J. Malde, G. W. Peterson, B. J. Schindler and P. Jones, Chem. Eng. Sci., 2012, 68, 376–382 CrossRef CAS PubMed.
- M. Kubo, K. Ishiyama, A. Shimojima and T. Okubo, Microporous Mesoporous Mater., 2012, 147, 194–199 CrossRef PubMed.
- C. B. Vidal, A. L. Barros, C. P. Moura, A. C. A. DeLima, F. S. Dias, L. C. G. Vasconcellos, P. B. A. Fechine and R. F. J. Nascimento, J. Colloid Interface Sci., 2011, 357, 466–473 CrossRef CAS PubMed.
- Y. Tang and K. Landskron, J. Phys. Chem. C, 2010, 114, 2494–2498 CAS.
- K. Sim, N. Lee, J. Kim, E. B. Cho, C. Gunathilake and M. Jaroniec, ACS Appl. Mater. Interfaces, 2015, 7, 6792–6802 CAS.
- B. Camarota, P. Ugliengo, E. Garrone, C. O. Arean, M. R. Delgado, S. Inagaki and B. Onida, J. Phys. Chem. C, 2008, 112, 19560–19567 CAS.
- G. N. Kalantzopoulos, A. Enotiadis, E. Maccallini, M. Antoniou, K. Dimos, A. Policicchio, E. Klontzas, E. Tylianakis, V. Binas, P. N. Trikalitis, R. G. Agostino, D. Gournis and G. E. Froudakis, Int. J. Hydrogen Energy, 2014, 39, 2104–2114 CrossRef CAS PubMed.
- D. Gust, T. A. Moore and A. L. Moore, Acc. Chem. Res., 2001, 34, 40–48 CrossRef CAS PubMed.
- M. S. Choi, T. Yamazaki, I. Yamazaki and T. Aida, Angew. Chem., Int. Ed., 2004, 43, 150–158 CrossRef CAS PubMed.
- A. Ajayaghosh and V. K. Praveen, Acc. Chem. Res., 2007, 40, 644–656 CrossRef CAS PubMed.
- H. Imahori, J. Phys. Chem. B, 2004, 108, 6130–6143 CrossRef CAS PubMed.
- M. H. V. Huynh, D. M. Dattelbaum and T. J. Meyer, Coord. Chem. Rev., 2005, 249, 457–483 CrossRef CAS PubMed.
- S. Inagaki, O. Ohtani, Y. Goto, K. Okamoto, M. Ikai, K. Yamanaka, T. Tani and T. Okada, Angew. Chem., Int. Ed., 2009, 48, 4042–4046 CrossRef CAS PubMed.
- H. Takeda, Y. Goto, Y. Maegawa, T. Ohsuna, T. Tani, K. Matsumoto, T. Shimad and S. Inagaki, Chem. Commun., 2009, 6032–6034 RSC.
- N. Mizoshita, Y. Goto, T. Tani and S. Inagaki, Adv. Mater., 2009, 21, 4798–4801 CrossRef CAS PubMed.
- N. Mizoshita, Y. Goto, Y. Maegawa, T. Tani and S. Inagaki, Chem. Mater., 2010, 22, 2548–2554 CrossRef CAS.
- P. N. Minoofar, B. S. Dunn and J. I. Zink, J. Am. Chem. Soc., 2005, 127, 2656–2665 CrossRef CAS PubMed.
- X. Zhang, M. A. Ballem, Z. J. Hu, P. Bergman and K. Uvdal, Angew. Chem., Int. Ed., 2011, 50, 5729–5733 CrossRef CAS PubMed.
- L. Grosch, Y. J. Lee, F. Hoffmann and M. Froba, Chem.–Eur. J., 2015, 21, 331–346 CrossRef PubMed.
- H. Inoue, T. Shimada, Y. Kou, Y. Nabetani, D. Masui, S. Takagi and H. Tachibana, ChemSusChem, 2011, 4, 173–179 CAS.
- J. H. Alstrum-Acevedo, M. K. Brennaman and T. J. Meyer, Inorg. Chem., 2005, 44, 6802–6827 CrossRef CAS PubMed.
- V. Balzani, A. Credi and M. Venturi, ChemSusChem, 2008, 1, 26–58 CrossRef CAS PubMed.
- T. Hirai and M. Ota, Mater. Res. Bull., 2006, 41, 19–28 CrossRef CAS PubMed.
- M. Morishita, Y. Shiraishi and T. Hirai, J. Phys. Chem. B, 2006, 110, 17898–17905 CrossRef CAS PubMed.
- H. Takeda, M. Ohashi, Y. Goto, T. Ohsuna, T. Tani and S. Inagaki, Chem.–Eur. J., 2014, 20, 9130–9136 CrossRef CAS PubMed.
- H. Takeda, M. Ohashi, T. Tani, O. Ishitani and S. Inagaki, Inorg. Chem., 2010, 49, 4554–4559 CrossRef CAS PubMed.
- M. Ohashi, M. Aoki, K. Yamanaka, K. Nakajima, T. Ohsuna, T. Tani and S. Inagaki, Chem.–Eur. J., 2009, 15, 13041–13046 CrossRef CAS PubMed.
- Y. Ueda, H. Takeda, T. Yui, K. Koike, Y. Goto, S. Inagaki and O. Ishitani, ChemSusChem, 2015, 8, 439–442 CrossRef CAS PubMed.
- Y. Lim, Y. S. Park, Y. Kang, D. Y. Jang, J. H. Kim, J. J. Kim, A. Sellinger and D. Y. Yoon, J. Am. Chem. Soc., 2011, 133, 1375–1382 CrossRef CAS PubMed.
- H. Xia, M. Li, D. Lu, C. Zhang, W. Xie, X. D. Liu, B. Yang and Y. Ma, Adv. Funct. Mater., 2007, 17, 1757–1764 CrossRef CAS PubMed.
- M. M. Shi, J. J. Lin, Y. W. Shi, M. Ouyang, M. Wang and H. Z. Chen, Mater. Chem. Phys., 2009, 115, 841–845 CrossRef CAS PubMed.
- J. W. Kang, D. S. Lee, H. D. Park, Y. S. Park, J. W. Kim, W. I. Jeong, K. M. Yoo, K. Go, S. H. Kim and J. J. Kim, J. Mater. Chem., 2007, 17, 3714–3719 RSC.
- T. Yamanari, T. Taima, J. Sakai, J. Tsukamoto and Y. Yoshida, Jpn. J. Appl. Phys., 2010, 49, 01AC02 CrossRef.
- N. Mizoshita, M. Ikai, T. Tani and S. Inagaki, J. Am. Chem. Soc., 2009, 131, 14225–14227 CrossRef CAS PubMed.
- S. Hamoudi, S. Royer and S. Kaliaguine, Microporous Mesoporous Mater., 2004, 71, 17–25 CrossRef CAS PubMed.
- Y. Guo, A. Mylonakis, Z. Zhang, G. Yang, P. I. Lelkes, S. Che, Q. Lu and Y. Wei, Chem.–Eur. J., 2008, 14, 2909–2917 CrossRef CAS PubMed.
- Y. Lu, H. Fan, N. Doke, D. A. Loy, R. A. Assink, D. A. LaVan and C. J. Brinker, J. Am. Chem. Soc., 2000, 122, 5258–5261 CrossRef CAS.
- B. D. Hatton, K. Landskron, W. Whitnall, D. D. Perovic and G. A. Ozin, Adv. Funct. Mater., 2005, 15, 823–829 CrossRef CAS PubMed.
- W. Wang, D. Grozea, A. Kim, D. D. Perovic and G. A. Ozin, Adv. Mater., 2010, 22, 99–102 CrossRef CAS PubMed.
- W. Wang, D. Grozea, S. Kohli, D. D. Perovic and G. A. Ozin, ACS Nano, 2011, 5, 1267–1275 CrossRef CAS PubMed.
- M. Seino, W. Wang, J. E. Lofgreen, D. P. Puzzo, T. Manab and G. A. Ozin, J. Am. Chem. Soc., 2011, 133, 18082–18085 CrossRef CAS PubMed.
- K. Landskron, B. D. Hatton, D. D. Perovic and G. A. Ozin, Science, 2003, 302, 266–269 CrossRef CAS PubMed.
- F. Goethals, M. R. Baklanov, I. Ciofi, C. Detavernier, P. V. D. Voort and I. V. Driessch, Chem. Commun., 2012, 48, 2797–2799 RSC.
- F. Goethals, I. Ciofi, O. Madia, K. Vanstreels, M. R. Baklanov, C. Detavernier, P. V. D. Voort and I. V. Driessche, J. Mater. Chem., 2012, 22, 8281–8286 RSC.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, immobilized lipase particles 150 mg, phosphate buffer pH 7.0 and temperature 30 °C. As a result a maximum biodiesel conversion to 95.8% was obtained via esterification of oleic acid with ethanol, whereas the biodiesel yield could be maintained at 88.6% after LP@PE was used 15 times. This significant enhanced activity can be explained due to the considerably increased interfacial area of the Pickering emulsion droplets, which can improve the mass transfer of reactants and the accessibility of enzyme in the reaction system as can be seen in Fig. 5.130
1, immobilized lipase particles 150 mg, phosphate buffer pH 7.0 and temperature 30 °C. As a result a maximum biodiesel conversion to 95.8% was obtained via esterification of oleic acid with ethanol, whereas the biodiesel yield could be maintained at 88.6% after LP@PE was used 15 times. This significant enhanced activity can be explained due to the considerably increased interfacial area of the Pickering emulsion droplets, which can improve the mass transfer of reactants and the accessibility of enzyme in the reaction system as can be seen in Fig. 5.130

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, LP@PMO 150 mg, phosphate buffer pH 7.0, 30 °C and 200 rpm). (b) The highest specific activity of LP@PE, LP@PMO and native lipase in esterification reactions, reproduced from ref. 130 with permission from Elsevier.
1, LP@PMO 150 mg, phosphate buffer pH 7.0, 30 °C and 200 rpm). (b) The highest specific activity of LP@PE, LP@PMO and native lipase in esterification reactions, reproduced from ref. 130 with permission from Elsevier.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1















![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O on the acridone groups whereas N-methylated precursor functionalized PMO films were transparent, suggesting that capping of the amine group hindered the intermolecular hydrogen bonds and effectively suppressed aggregate formation during PMO films casting.228 Uvdal et al.232 have reported that ligands with different p-conjugation lengths and side chains show impact on the optical properties. Direct modification of the ligands by using hydrophobic alkyl chains has been proven to effectively stabilize the particles. Different metal ions, donors, and acceptors have been incorporated into the frameworks by one-step coordination-directed assembly. Experimental results clearly show that the optical properties of nanoscale light-harvesting metal–organic frameworks (NMOFs) can be enhanced and tuned by chemical manipulation of the inorganic and organic components in the frameworks. The development of photocatalytic systems linked with light-harvesting function is also important.234–240 Hirai et al.237 have immobilized CdS nanoparticles into PMOs to be used as photocatalyst for the generation of H2 from 2-propanol aqueous solution. It was shown that morphology, stability, and framework hydrophobicity have affected photocatalytic activity. On the other hand, Ti-functionalized PMO (Ti-PMO) was also found to be employed as a photocatalyst for selective olefin epoxidation.210 The reaction rate on the Ti-containing PMO catalysts is similar to that on Ti-containing pure silica due to the destabilization of these oxygen radicals on the hydrophobic surface of the Ti-PMO catalysts. It is well-known that the O3− radical is a crucial oxidant for epoxide formation which is more destabilized once O3− radical is on the hydrophobic Ti-PMO surface. On the other hand, the hydrophobic olefins also easily access the photoexcited Ti–O4 species on the hydrophobic Ti-PMOs surface. What happened then is that the destabilizations of the photoexcited Ti–O4 species and O3− radical counteract the enhanced access of olefins to the excited species, resulting in almost no improvement in the olefin conversion.238 In 2014, a visible-light harvesting antenna based on acridone groups functionalized PMO (Acd-PMO) was successfully synthesised using [RuII(bpy)32+] complex (bpy = 2,2′-bipyridyl) coupled with iridium oxide (IrOx) particles supported in the PMO meso channels framework as photosensitizer and catalyst, respectively.239 It was found that Acd-PMO absorbed visible light and funnelled the light energy into the Ru complex on the meso channels through excitation energy transfer. From this study, the reaction quantum yield was 0.34%, which was improved to 0.68% by the modifications of PMO.239 Inagaki and co-workers239 also reported surfactant-mediated PMO for an artificial photosynthesis system with three-dimensional organization of molecular parts, such as a light absorber and a Re-complex as the multi-electron catalyst. A rhenium (Re) bipyridine complex was chosen as a reaction centre as is a well-known two-electron-reduction photocatalyst for conversion of CO2 to CO. This is a very new conceptual design for enhancing the photocatalytic CO2 reduction of rhenium(I) complex placed in the mesochannels of PMO framework.
O on the acridone groups whereas N-methylated precursor functionalized PMO films were transparent, suggesting that capping of the amine group hindered the intermolecular hydrogen bonds and effectively suppressed aggregate formation during PMO films casting.228 Uvdal et al.232 have reported that ligands with different p-conjugation lengths and side chains show impact on the optical properties. Direct modification of the ligands by using hydrophobic alkyl chains has been proven to effectively stabilize the particles. Different metal ions, donors, and acceptors have been incorporated into the frameworks by one-step coordination-directed assembly. Experimental results clearly show that the optical properties of nanoscale light-harvesting metal–organic frameworks (NMOFs) can be enhanced and tuned by chemical manipulation of the inorganic and organic components in the frameworks. The development of photocatalytic systems linked with light-harvesting function is also important.234–240 Hirai et al.237 have immobilized CdS nanoparticles into PMOs to be used as photocatalyst for the generation of H2 from 2-propanol aqueous solution. It was shown that morphology, stability, and framework hydrophobicity have affected photocatalytic activity. On the other hand, Ti-functionalized PMO (Ti-PMO) was also found to be employed as a photocatalyst for selective olefin epoxidation.210 The reaction rate on the Ti-containing PMO catalysts is similar to that on Ti-containing pure silica due to the destabilization of these oxygen radicals on the hydrophobic surface of the Ti-PMO catalysts. It is well-known that the O3− radical is a crucial oxidant for epoxide formation which is more destabilized once O3− radical is on the hydrophobic Ti-PMO surface. On the other hand, the hydrophobic olefins also easily access the photoexcited Ti–O4 species on the hydrophobic Ti-PMOs surface. What happened then is that the destabilizations of the photoexcited Ti–O4 species and O3− radical counteract the enhanced access of olefins to the excited species, resulting in almost no improvement in the olefin conversion.238 In 2014, a visible-light harvesting antenna based on acridone groups functionalized PMO (Acd-PMO) was successfully synthesised using [RuII(bpy)32+] complex (bpy = 2,2′-bipyridyl) coupled with iridium oxide (IrOx) particles supported in the PMO meso channels framework as photosensitizer and catalyst, respectively.239 It was found that Acd-PMO absorbed visible light and funnelled the light energy into the Ru complex on the meso channels through excitation energy transfer. From this study, the reaction quantum yield was 0.34%, which was improved to 0.68% by the modifications of PMO.239 Inagaki and co-workers239 also reported surfactant-mediated PMO for an artificial photosynthesis system with three-dimensional organization of molecular parts, such as a light absorber and a Re-complex as the multi-electron catalyst. A rhenium (Re) bipyridine complex was chosen as a reaction centre as is a well-known two-electron-reduction photocatalyst for conversion of CO2 to CO. This is a very new conceptual design for enhancing the photocatalytic CO2 reduction of rhenium(I) complex placed in the mesochannels of PMO framework.