Precisely designed perylene bisimide-substituted polyethylene with a high glass transition temperature and an ordered architecture†
Wei Song,
Jianhua Wu,
Guangda Yang,
Huijing Han,
Meiran Xie* and
Xiaojuan Liao*
School of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200241, China. E-mail: mrxie@chem.ecnu.edu.cn; xjliao@chem.ecnu.edu.cn; Fax: +86 21 54340058/+86 21 54340105; Tel: +86 21 54340058/+86 21 54340105
First published on 3rd August 2015
Abstract
Acyclic diene metathesis polymerization of a structurally symmetrical perylene bisimide (PBI)-containing α,ω-diene has been performed, yielding an unsaturated polymer with increased molecular weight (Mn = 21.2–87.6 kDa) and decreased polydispersity index (PDI = 2.31–1.76) as the reaction time was prolonged. The subsequent hydrogenation of the as-synthesized polymer was readily accomplished, affording the desired polyethylene (PE) with a saturated backbone and precisely repeating substituted bulky PBI branches. This PBI-substituted PE derivative displayed high glass transition temperatures (Tg = 51.8–75.8 °C), a relatively wide range of light absorption (λ = 230–590 nm), and a highly ordered architecture, which should facilitate electron mobility and be suitable for utilization in optoelectronic devices. It can therefore serve as a superior model for simple construction of functional PE polymers with precisely repeating bulky branches and a soluble PBI polymer with an ordered architecture.
Introduction
Polyethylene (PE)-based polymers have been used in a wide variety of commodity applications and are considered as one of the most important commercial thermoplastic products in the world.1 The physical properties of these polymers can be widely varied by manipulating their microstructural parameters, in particular the length and placement of branches along the polymer chain.2 Despite their commercial importance, the lack of functional groups in their structure is a common problem. An effective approach to providing functionality to polyolefins is the attachment of functional groups in the polymer main chain or side chain. In this way, a collective research effort has been dedicated to the introduction of functional groups such as aryl ether units,3 hydroxyl and alkyl groups,4 and imidazolium pendants,5 on a polyolefin architecture to adjust the chemical composition and designable performance of PE-based polymers. However, the introduction of bulky aryl chromophore moieties relevant for traditional PE-based polymers is still rare.Perylene 3,4:9,10-tetracarboxylic acid bisimide (PBI) derivatives, which were considered for use as colorants in the early development of perylene dyes, are among the most intensively studied functional building blocks based on macromolecular approaches. Afterwards, it became evident that because of the n-type semiconductivity, fruitful optical properties, and excellent thermal stability of PBI derivatives, and because it is easy to chemically functionalize them,6 these derivatives are well-suited to serve as building blocks not only in the field of organic solar cells, including bulk-heterojunction solar cells and dye-sensitized solar cells,7 but also for the construction of supramolecules; PBI derivatives are particularly suitable in this regard since functional groups can be flexibly introduced to either the terminal imide moiety or the bay positions of the perylene core to create structurally complex and multifunctional molecules.8–10
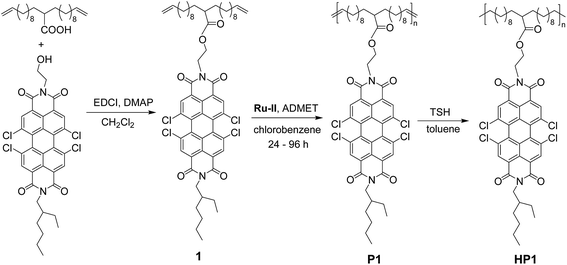
Incorporating PBI moieties on the PE side chain may endow the polymer with enhanced toughness, outstanding optical properties, and improved thermal properties, thus making it an ideal candidate as an n-type elastic polymer. Another reason for choosing PBI as the chromophore is the considerable body of work regarding its supramolecular interactions such as π-stacking with other PBI moieties or with other chromophores.11 Acyclic diene metathesis (ADMET) polymerization serves as a powerful and broadly applicable methodology to introduce periodic pendant groups at specific positions in PE-based polymers. The periodic sequence encoded in the final polymer can be successfully predetermined through a tailor-made diene monomer via a simple ADMET polymerization. Herein, a PBI-containing α,ω-diene monomer was synthesized, and then a PE derivative possessing PBI branches on every 21st carbon was obtained using ADMET polymerization followed by application of a hydrogenation methodology. This product was observed to have a highly ordered architecture, good thermal stability, and attractive optoelectronic properties. To our knowledge, it is the first well-defined PE derivative containing sequential PBI branches by ADMET polymerization, and this type of chromophore-containing polymer has the potential to become a widely used polyolefin.
Experimental
Materials
[1,3-Bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene] benzylidene ruthenium dichloride (Grubbs' second-generation catalyst, Ru-II) and 4-methylbenzenesulfonhydrazide (TSH) were obtained from Aldrich. Ethyl vinyl ether (stabilized with 0.1% N,N-diethylaniline) was purchased from Acros. 2,6-Di-tert-butyl-4-methylphenol (BHT), 2-aminoethanol, and 2-ethylhexylamine were purchased from Shanghai Chemical Reagents Co., Ltd. 1,6,7,12-Tetrachloroperylene-3,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9,10-tetracarboxylic dianhydride (TCPTCDA) was purchased from commercial sources at analytical grade and used without further purification. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimidehydrochloride (EDCI·HCl) and 4-dimethylaminopyridine (DMAP) were purchased from Energy Chemical. 2-Undec-10-enyltridec-12-enoic acid was synthesized according to the published procedure.12 All reactions were carried out under dry nitrogen atmosphere using standard Schlenk-line techniques. Solvents were distilled over drying agents under nitrogen prior to use, including dichloromethane (CH2Cl2), toluene, and chlorobenzene from calcium hydride.
9,10-tetracarboxylic dianhydride (TCPTCDA) was purchased from commercial sources at analytical grade and used without further purification. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimidehydrochloride (EDCI·HCl) and 4-dimethylaminopyridine (DMAP) were purchased from Energy Chemical. 2-Undec-10-enyltridec-12-enoic acid was synthesized according to the published procedure.12 All reactions were carried out under dry nitrogen atmosphere using standard Schlenk-line techniques. Solvents were distilled over drying agents under nitrogen prior to use, including dichloromethane (CH2Cl2), toluene, and chlorobenzene from calcium hydride.
Characterization
1H (500 MHz) and 13C (125 MHz) NMR spectra were recorded using tetramethylsilane as an internal standard in CDCl3 on a Bruker DPX spectrometer. The melting point was determined by using a Micro melting point apparatus (Yanoco). FT-IR spectra were recorded on a Nicolet Nexus 670 in the region of 4000–400 cm−1 using KBr pellets. The high resolution mass spectroscopy (HRMS) was measured by a Bruker QTOF micromass spectrometer. IR spectra were recorded by a PerkinElmer Spectrum One FTIR spectrophotometer. UV-Vis absorption spectra were measured on a UV-1800 spectrometer. Gel permeation chromatography (GPC) was used to calculate relative number-and weight-average molecular weights (Mn and Mw) and the polydispersity index (PDI); the equipment used here included a Waters 1515 Isocratic HPLC pump, a Waters 2414 refractive index detector, and a set of Waters Styragel columns (7.8 × 300 mm, 5 mm bead size; 103, 104, and 105 Å pore size). The sample for transmission electron microscopy (TEM) was prepared by depositing a drop of the solution (1 mg mL−1) on a carbon-coated Cu grid, and TEM images were recorded on JEOL2100F microscopes operating at 120 kV. X-ray diffraction (XRD) analysis was performed on a Bruker D8 ADVANCE instrument using Cu Kα radiation (k = 1.5418 Å). An atomic force microscopy (AFM) observation was made using an SPM AJ-III atomic force microscope at a measuring rate of 1.0005 Hz in tapping mode, and the AFM images were obtained at room temperature in air. The sample for the AFM measurement was prepared by drop-coating a CH2Cl2 solution at 0.0025 mg mL−1 on a freshly cleaved mica surface, which was then air-dried at room temperature. The sample was examined at least twice under the same conditions, and the images were found to be very reproducible. Differential scanning calorimetry (DSC) was performed on a Netzsch 204F1 in a nitrogen atmosphere. An indium standard was used for temperature and enthalpy calibrations. All of the samples were first heated from −50 to 200 °C and held at this temperature for 3 min to eliminate any thermal history, and then they were cooled to room temperature and heated again from −50 to 200 °C at a heating or cooling rate of 10 °C min−1. Thermal gravimetric analysis (TGA) was performed using an SDTA851e/SF/1100 TGA instrument under nitrogen flow at a heating rate of 10 °C min−1 from 50 to 800 °C. Cyclic voltammetry (CV) was carried out with an Autolab PGSTAT12 potentiostat from Eco Chemie coupled to an electrochemical cell with three electrodes. The scan rate was 100 mV s−1. A glassy carbon electrode was used as the working electrode, a Pt wire as the counter electrode, and Ag/AgCl was used as the reference electrode. 0.1 M Bu4NPF6 of CH3CN solution was used as the supporting electrolyte, and Fc+/Fc was used as the reference.Synthesis of N-2-ethylhexyl-N′-2-hydroxyethyl-1,6,7,12-tetrachloroperylene-3,4:9,10-tetracarboxylic bisimide (TCPBI-OH)
To a suspension of TCPTCDA (2.65 g, 5 mmol) in 30 mL of toluene, 2-ethylhexylamine (0.69 g, 5.4 mmol) and ethanolamine (0.33 g, 5.4 mmol) were added and stirred at reflux under a nitrogen atmosphere for 24 h. After being cooled to room temperature, 100 mL of CH2Cl2 was added to obtain a red precipitate. After filtration, the CH2Cl2 phase was evaporated to remove solvent, and purified by column chromatography on silica gel using CH2Cl2/EtOAc (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as the eluent. The product TCPBI–OH was obtained as a dark red powder (1.08 g, yield 25%). 1H NMR (CDCl3, ppm): δ 8.68 (s, 4H, pery), 4.56–4.39 (t, 2H, NCH2CH2OH), 4.19–4.06 (t, 2H, NCH2CH2OH), 4.04–3.93 (d, J = 8.1 Hz, 2H, NCH2CH), 1.95 (s, 1H, NCH2CH2OH), 1.89–1.69 (m, 1H, NCH2CHCH2), 1.43–1.13 (m, 8H, CH2 on ethylhexyl), 1.01–0.80 (m, 6H, CH3); 13C NMR (CDCl3, ppm): δ 163.13, 162.70, 135.54, 135.37, 133.24, 133.07, 131.44, 128.94, 128.50, 123.50, 122.94, 61.11, 44.61, 42.87, 38.11, 30.71, 28.70, 23.90, 23.03, 14.21, 10.73.
1 v/v) as the eluent. The product TCPBI–OH was obtained as a dark red powder (1.08 g, yield 25%). 1H NMR (CDCl3, ppm): δ 8.68 (s, 4H, pery), 4.56–4.39 (t, 2H, NCH2CH2OH), 4.19–4.06 (t, 2H, NCH2CH2OH), 4.04–3.93 (d, J = 8.1 Hz, 2H, NCH2CH), 1.95 (s, 1H, NCH2CH2OH), 1.89–1.69 (m, 1H, NCH2CHCH2), 1.43–1.13 (m, 8H, CH2 on ethylhexyl), 1.01–0.80 (m, 6H, CH3); 13C NMR (CDCl3, ppm): δ 163.13, 162.70, 135.54, 135.37, 133.24, 133.07, 131.44, 128.94, 128.50, 123.50, 122.94, 61.11, 44.61, 42.87, 38.11, 30.71, 28.70, 23.90, 23.03, 14.21, 10.73.
Synthesis of α,ω-diene monomer with the TCPBI pendant group (1)
The compound TCPBI–OH (3.28 g, 4.8 mmol) was first dissolved in 50 mL of anhydrous CH2Cl2. To this solution, 2-undec-10-enyltridec-12-enoic acid (1.46 g, 4 mmol), EDCI·HCl (0.92 g, 5.8 mmol) and DMAP (0.1 g, 0.8 mmol) were added under nitrogen atmosphere in an ice-water bath and stirred for 2 h. Then the reaction proceeded at room temperature and was monitored by using TLC. After 2 days, the mixture was washed with dilute hydrochloric acid (5 × 30 mL), followed by water, and dried with anhydrous MgSO4. After filtration and removing the solvent, crude product was purified by using column chromatography on a silica gel using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH2Cl2/petroleum ether as the eluent. The α,ω-diene monomer 1 was obtained as a red powder (3.82 g, yield 95%). 1H NMR (CDCl3, ppm): δ 8.67 (s, 4H, pery), 5.81–5.69 (m, 2H, CH2
1 CH2Cl2/petroleum ether as the eluent. The α,ω-diene monomer 1 was obtained as a red powder (3.82 g, yield 95%). 1H NMR (CDCl3, ppm): δ 8.67 (s, 4H, pery), 5.81–5.69 (m, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 4.98–4.85 (m, 4H, CH2
CHCH2), 4.98–4.85 (m, 4H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2), 4.59–4.41 (m, 4H, NCH2CH2O), 4.22–4.07 (t, 2H, NCH2CHCH2), 2.32–2.20 (m, 1H, NCH2CHCH2), 2.00–1.87 (m, 5H, CH2
CHCH2), 4.59–4.41 (m, 4H, NCH2CH2O), 4.22–4.07 (t, 2H, NCH2CHCH2), 2.32–2.20 (m, 1H, NCH2CHCH2), 2.00–1.87 (m, 5H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2 + CH2CHCOO), 1.55–1.01 (m, 40H, CH2 on alkyl chain), 1.00–0.78 (m, 6H, CH3); 13C NMR (CDCl3, ppm): δ 176.26, 162.59, 162.20, 139.14, 135.49, 135.28, 133.02, 131.42, 128.88, 128.58, 123.34, 122.91, 114.10, 61.27, 45.52, 44.67, 39.55, 37.97, 33.82, 32.11, 30.71, 29.59, 29.52, 29.43, 29.11, 28.88, 28.66, 27.39, 14.12, 10.58; IR (cm−1): 3062, 2921, 2852, 1706, 1665, 1584, 1497, 1389, 1338, 1288, 1236, 1154, 1053, 991, 908, 845, 806, 746, 684, 545; HR-MS: calcd for C59H71Cl4N2O6Na [M + Na]+: 1069.0168, found: 1069.0172.
CHCH2 + CH2CHCOO), 1.55–1.01 (m, 40H, CH2 on alkyl chain), 1.00–0.78 (m, 6H, CH3); 13C NMR (CDCl3, ppm): δ 176.26, 162.59, 162.20, 139.14, 135.49, 135.28, 133.02, 131.42, 128.88, 128.58, 123.34, 122.91, 114.10, 61.27, 45.52, 44.67, 39.55, 37.97, 33.82, 32.11, 30.71, 29.59, 29.52, 29.43, 29.11, 28.88, 28.66, 27.39, 14.12, 10.58; IR (cm−1): 3062, 2921, 2852, 1706, 1665, 1584, 1497, 1389, 1338, 1288, 1236, 1154, 1053, 991, 908, 845, 806, 746, 684, 545; HR-MS: calcd for C59H71Cl4N2O6Na [M + Na]+: 1069.0168, found: 1069.0172.
ADMET polymerization of monomer
A 15 mL Schlenk tube was charged with monomer 1 (210 mg, 0.2 mmol) dissolved in 0.8 mL of chlorobenzene. In another 10 mL Schlenk tube, Ru-II (3.4 mg, 4 μmol) was dissolved in 0.2 mL of chlorobenzene. After being degassed in three freeze-vacuum-thaw cycles, the Ru-II catalyst solution was then injected into the monomer solution via a syringe under vigorous stirring at 50 °C. The reaction was allowed to proceed for 24–96 h followed by a slow purge of nitrogen to drive off the ethylene condensate. During this procedure, a second aliquot of the mixture was withdrawn from the tube via a syringe and 1 mol% Ru-II was added at the predetermined time intervals to monitor the metathesis reaction by GPC, and the resulting mixture gradually became viscous as the molecular weight of the polymer increased and as the solvent chlorobenzene partially evaporated. The polymerization was finally quenched by adding an excess of ethyl vinyl ether with stirring for another 30 min. The mixture was poured into 20 mL of petroleum ether and the precipitate was isolated by filtration, dried under vacuum at 60 °C for 24 h to give the unsaturated polymer P1 as a dark-red solid in high yields. 1H NMR (CDCl3, ppm): δ 8.67 (s, pery), 5.30 (s, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH on backbone), 4.64–4.41 (m, NCH2CH2O), 4.25–4.05 (m, NCH2CHCH2), 2.35–2.19 (m, NCH2CHCH2), 2.08–1.79 (t, CH2
CH on backbone), 4.64–4.41 (m, NCH2CH2O), 4.25–4.05 (m, NCH2CHCH2), 2.35–2.19 (m, NCH2CHCH2), 2.08–1.79 (t, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2 + CH2CHCOO), 1.78–1.05 (m, CH2 on alkyl chain), 1.02–0.84 (m, CH3 on alkyl chain); 13C NMR (CDCl3, ppm): δ 176.44, 162.53, 162.19, 135.49, 135.36, 133.03, 133.01, 131.38, 131.42, 130.24, 129.79, 128.79, 128.46, 123.35, 123.31, 122.93, 61.07, 44.86, 44.60, 39.74, 37.95, 32.58, 32.16, 30.66, 28.61, 27.42, 27.19, 24.00, 23.04, 14.13, 10.56; IR (cm−1): 3062, 2921, 2852, 1707, 1665, 1584, 1497, 1438, 1381, 1340, 1285, 1232, 1132, 1053, 962, 907, 861, 806, 743, 684, 545. GPC: Mn = 21.2–87.6 kDa, PDI = 2.31–1.76.
CHCH2 + CH2CHCOO), 1.78–1.05 (m, CH2 on alkyl chain), 1.02–0.84 (m, CH3 on alkyl chain); 13C NMR (CDCl3, ppm): δ 176.44, 162.53, 162.19, 135.49, 135.36, 133.03, 133.01, 131.38, 131.42, 130.24, 129.79, 128.79, 128.46, 123.35, 123.31, 122.93, 61.07, 44.86, 44.60, 39.74, 37.95, 32.58, 32.16, 30.66, 28.61, 27.42, 27.19, 24.00, 23.04, 14.13, 10.56; IR (cm−1): 3062, 2921, 2852, 1707, 1665, 1584, 1497, 1438, 1381, 1340, 1285, 1232, 1132, 1053, 962, 907, 861, 806, 743, 684, 545. GPC: Mn = 21.2–87.6 kDa, PDI = 2.31–1.76.
Hydrogenation of the unsaturated polymer
The samples P1a, P1c, and P1d obtained under reaction time of 24–96 h were taken as the starting materials for a further investigation. Typically, P1d (180 mg, 1.8 mmol), TSH (101 mg, 0.5 mmol), and BHT (50 mg) were weighed and added into a 100 mL Schlenk flask. Then, 10 mL of toluene was added to obtain a dark-red suspension. The flask was connected to a reflux condenser that was equipped with a bubbling tube and placed in an oil bath at 120 °C under vigorous stirring. After 6 h, the reaction was stopped on cooling to room temperature to add another equal supply of TSH, and the reaction was allowed to proceed for another 6 h at 130 °C. The mixture was poured into petroleum ether, and the precipitated solid was redissolved in 1 mL of CH2Cl2 to give a viscous solution, which was added dropwise into 20 mL of cold methanol to obtain an orange precipitate. After filtration and vacuum dryness, the saturated polymer HP1d was obtained in 98% yield. 1H NMR (500 MHz, CDCl3, ppm): δ 8.67 (s, pery), 4.74–4.34 (m, NCH2CH2O), 4.29–3.75 (m, NCH2CHCH2), 2.61–2.12 (m, NCH2CHCH2), 2.08–1.82 (t, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCH2 + CH2CHCOO), 1.78–0.52 (m, CH2 + CH3 on alkyl); 13C NMR (125 MHz, CDCl3, ppm): δ 176.32, 162.56, 162.22, 135.49, 135.36, 133.05, 131.51, 131.43, 128.81, 128.43, 128.17, 123.35, 123.30, 122.94, 61.15, 45.59, 44.67, 39.68, 38.04, 32.20, 28.59, 27.44, 24.03, 23.04, 14.08, 10.57; IR (cm−1): 3059, 2923, 2852, 1711, 1670, 1584, 1443, 1381, 1340, 1285, 1233, 1149, 1063, 917, 846, 808, 742, 684, 590, 542. GPC: Mn = 87.4 kDa, PDI = 1.62.
CHCH2 + CH2CHCOO), 1.78–0.52 (m, CH2 + CH3 on alkyl); 13C NMR (125 MHz, CDCl3, ppm): δ 176.32, 162.56, 162.22, 135.49, 135.36, 133.05, 131.51, 131.43, 128.81, 128.43, 128.17, 123.35, 123.30, 122.94, 61.15, 45.59, 44.67, 39.68, 38.04, 32.20, 28.59, 27.44, 24.03, 23.04, 14.08, 10.57; IR (cm−1): 3059, 2923, 2852, 1711, 1670, 1584, 1443, 1381, 1340, 1285, 1233, 1149, 1063, 917, 846, 808, 742, 684, 590, 542. GPC: Mn = 87.4 kDa, PDI = 1.62.
Results and discussion
Synthesis of the diene monomer
The diene monomer 1 bearing the PBI pendant was synthesized in a simple way, and the synthetic route and chemical structure of monomer 1 is depicted in Scheme 1. First, the imidization reaction of commercially available starting material TCPTCDA with a mixture of 2-aminoethanol and 2-ethylhexylamine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol mol−1) was accomplished, giving the unsymmetrical compound TCPBI–OH with a branched alkyl and a reactive hydroxyethyl group13 (Scheme S1, ESI†). Then the monomer 1 with a spacer of 18 methylenes between diene end groups was easily obtained by a subsequent esterification reaction of TCPBI–OH with 2-undec-10-enyltridec-12-enoic acid12 in CH2Cl2 solution at room temperature. The crude product was purified by utilizing column chromatography on silica gel using CH2Cl2/petroleum ether (1
1 mol mol−1) was accomplished, giving the unsymmetrical compound TCPBI–OH with a branched alkyl and a reactive hydroxyethyl group13 (Scheme S1, ESI†). Then the monomer 1 with a spacer of 18 methylenes between diene end groups was easily obtained by a subsequent esterification reaction of TCPBI–OH with 2-undec-10-enyltridec-12-enoic acid12 in CH2Cl2 solution at room temperature. The crude product was purified by utilizing column chromatography on silica gel using CH2Cl2/petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as the eluent to give monomer 1 as a red powder.
1 v/v) as the eluent to give monomer 1 as a red powder.
1H, 13C NMR, and HR-MS spectroscopy were employed to confirm the chemical structure of the compound. The 1H NMR spectrum (Fig. 1A) indicates the presence of an aromatic proton on the PBI branch at 8.67 ppm and the terminal double protons at both 5.81–5.69 and 4.98–4.85 ppm, and the integration values of the peaks at 8.67, 5.81–5.69, and 4.98–4.85 ppm were nearly 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, illustrating that the TCPBI moiety had been successfully attached to 2-undec-10-enyltridec-12-enoic acid. Meanwhile, monomer 1 was also characterized by 13C NMR spectroscopy as shown in Fig. 1E. The strong resonances of C
1, illustrating that the TCPBI moiety had been successfully attached to 2-undec-10-enyltridec-12-enoic acid. Meanwhile, monomer 1 was also characterized by 13C NMR spectroscopy as shown in Fig. 1E. The strong resonances of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O on the ester and imide groups from monomer 1 were observed at about 176.26, 162.59, and 162.20 ppm. Simultaneously, the resonance signals of carbons at 135.49, 135.28, 133.02, 131.42, 128.88, 128.58, 123.34, and 122.91 ppm, corresponding to the chemical structure of the perylene branch, were still present. The other two peaks at 139.14 and 114.10 ppm were assigned to the unsaturated carbon on the terminal double bond. The IR spectrum of monomer 1 (Fig. 2) showed the characteristic stretching of the PBI branch, with peaks at 1706 and 1665 cm−l. The shoulder peaks at 2921 and 2852 cm−1 were assigned to the saturated CH2 group, and the characteristic absorption bands at 1389, 1235, 1053, and 684 cm−1 were attributed to the stretching vibrations of C–N, C
O on the ester and imide groups from monomer 1 were observed at about 176.26, 162.59, and 162.20 ppm. Simultaneously, the resonance signals of carbons at 135.49, 135.28, 133.02, 131.42, 128.88, 128.58, 123.34, and 122.91 ppm, corresponding to the chemical structure of the perylene branch, were still present. The other two peaks at 139.14 and 114.10 ppm were assigned to the unsaturated carbon on the terminal double bond. The IR spectrum of monomer 1 (Fig. 2) showed the characteristic stretching of the PBI branch, with peaks at 1706 and 1665 cm−l. The shoulder peaks at 2921 and 2852 cm−1 were assigned to the saturated CH2 group, and the characteristic absorption bands at 1389, 1235, 1053, and 684 cm−1 were attributed to the stretching vibrations of C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O–C, and the out-of-plane bending vibration of the C
O, and C–O–C, and the out-of-plane bending vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H group, respectively. Besides, the vibration of the terminal CH
C–H group, respectively. Besides, the vibration of the terminal CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 group at 990 cm−1 was also observed. Furthermore, the molecular weight of monomer 1 by HR-MS analysis was in good accordance with the calculated value. All of these points assured that monomer 1 was successfully synthesized.
CH2 group at 990 cm−1 was also observed. Furthermore, the molecular weight of monomer 1 by HR-MS analysis was in good accordance with the calculated value. All of these points assured that monomer 1 was successfully synthesized.
ADMET polymerization of monomer and hydrogenation of polymer
Ru-II was selected as the catalyst for ADMET polymerization on the basis of its high catalytic performance. The ADMET polymerization of monomer 1 was conducted in chlorobenzene at 60 °C for 24–96 h under a continuous nitrogen purge to remove the generated ethylene byproduct. During this procedure, an aliquot of the reactive mixture was withdrawn from the tube via a syringe and 1 mol% Ru-II was added at the predetermined 24 h intervals to monitor the metathesis reaction by GPC. After purification, the unsaturated polymer P1(a–d) was obtained as a dark red solid.Exhaustive hydrogenation of the unsaturated polymer (P1) afforded saturated PE incorporating PBI branches on every 21st carbon. Our previous report on the synthesis of a saturated hybrid polymer14 illustrated that the hydrogenation degree of double bonds achieved by TSH in 4 h was reasonably high, but some unsaturation still remained. Consequently, to ensure complete hydrogenation, two batch feedings of TSH were used at a time interval of 6 h. After hydrogenation, the saturated periodic polymer HP1 was obtained as an orange solid.
The representative unsaturated P1d was characterized by 1H and 13CNMR spectroscopy. As compared with monomer 1, the signals at 5.81–5.69 ppm and 4.98–4.85 ppm in the 1H NMR spectrum (Fig. 1A) as well as at 139.14 and 114.10 ppm in the 13C NMR spectrum (Fig. 1E) assigned to the terminal alkenes (a, b) disappeared after ADMET polymerization, while the protons of newly formed internal alkenes (b′) on the polymer main chain appeared at 5.30 ppm in the 1H NMR spectrum (Fig. 1B). Meanwhile, the signals for terminal alkenes (a′), which should appear at about 5.0 and 5.7 ppm, could not be clearly detected, indicating that high-molecular-weight P1d was obtained. Also, the peaks at 130.24 and 129.79 ppm in the 13C NMR spectrum (Fig. 1E) were attributed to the internal trans and cis double bonds.4 The structure was further confirmed by IR spectroscopy (Fig. S1, ESI†): when comparing the IR spectrum of polymer P1d with that of monomer 1 (Fig. 2), we clearly observed that the absorption bands exhibited no significant change, except for the disappearance of the terminal double bonds (990 cm−1); meanwhile, a new absorption peak appeared at 964 cm−1, and the peak at 684 cm−1 strengthened, which contributed to evidence for the presence of the trans and cis double bonds on the polymer backbone, respectively. This is consistent with the 13C NMR results of the cis/trans double bonds for unsaturated P1d.
After exhaustive hydrogenation, as can be seen from the NMR spectra of saturated HP1d (Fig. 1C and F), the signals of the internal alkenic protons (5.30, 130.24, and 129.79 ppm) in P1d disappeared, indicating that a complete hydrogenation was achieved using the above-mentioned protocol. In particular, hydrolysis of the ester groups, which may be caused by the generated p-toluenesulfonic acid, did not occur, as the aromatic proton (8.67 ppm) on HP1d still remained.
The IR spectrum for saturated HP1d is shown in Fig. 2. The absorption bands were observed to be almost the same as for P1d except for the disappearance of the trans double bonds at 964 cm−1 and the weakening of the cis double bonds at 684 cm−1 (Fig. 2). Therefore, we concluded that the unsaturated P1d was completely converted to the saturated HP1d.
The resulting unsaturated polymer P1 showed number–average molecular weight (Mn) values ranging from 21.2 kDa to 98.7 kDa, with a reasonable and reduced PDI ranging from 2.31 to 1.76 (Table 1) as the reaction time was prolonged. Additionally, the GPC traces of polymers obtained from different reaction time are shown in Fig. 3. As the polymerization progressed, the elution curves gradually shifted to a higher molecular weight region and the PDIs also become narrower, indicating an increase in the number of repeated monomeric units, which was in good agreement with the gradual polymerization. In addition, the Mn value of the saturated periodic HP1d was essentially the same as that of its precursor P1d, which showed with sufficient confidence that no degradation occurred under this reaction condition.
| Polymer | t (h) | Yieldb (%) | Mnc (kDa) | PDIc | Tdd (°C) | Tge (°C) |
|---|---|---|---|---|---|---|
a Reaction conditions: [M]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C]0 = 50 [C]0 = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, [M]0 = 0.50 mol L−1, polymerization was run in chlorobenzene with Ru-II catalyst at 60 °C.b Obtained gravimetrically after purification from the dried polymer.c Mn was determined by GPC in THF relative to monodispersed polystyrene standards.d Decomposition temperature at 5% weight loss determined by TGA.e Glass transition temperature determined by DSC.f Hydrogenation time of unsaturated polymer. 1, [M]0 = 0.50 mol L−1, polymerization was run in chlorobenzene with Ru-II catalyst at 60 °C.b Obtained gravimetrically after purification from the dried polymer.c Mn was determined by GPC in THF relative to monodispersed polystyrene standards.d Decomposition temperature at 5% weight loss determined by TGA.e Glass transition temperature determined by DSC.f Hydrogenation time of unsaturated polymer. |
||||||
| P1a | 24 | 88.5 | 21.2 | 2.31 | 263 | 59.2 |
| P1b | 48 | 97.2 | 32.7 | 1.95 | — | — |
| P1c | 72 | 98.0 | 49.5 | 1.81 | — | 76.5 |
| P1d | 96 | 98.7 | 87.6 | 1.76 | 367 | 80.4 |
| HP1a | 12f | 98.2 | 21.0 | 2.11 | 295 | 51.8 |
| HP1c | 12f | 98.0 | 49.3 | 1.80 | — | 64.5 |
| HP1d | 12f | 97.5 | 87.4 | 1.62 | 385 | 75.8 |
Thin film morphology
TEM images for thin films of P1d and HP1d using the drop-casting method are shown in Fig. S2 and S3 (ESI†). Their particle nature was clearly visible from the TEM images at low magnification. By enlarging the particle image, the HR-TEM investigation of polymers P1d and HP1d gave an indication of the long-range ordered architecture (Fig. 4a1 and b1), and the selected area electron diffraction (SAED) patterns (Fig. 4a2 and b2) of P1d and HP1d acquired during the TEM analysis confirmed the ordered architecture of the polymers. This highly regular architecture was caused by the typically crystalline nature of the PBI branches rather than of the PE backbones,15 which was also reflected by the XRD characterization. The primary diffraction peaks in the XRD patterns (Fig. S4, ESI†) were observed at 2θ = 0.8° and 19.4° for P1d with d values of 107.7 and 4.5 Å, respectively, and at 2θ = 1.02° and 20.8° for HP1d with d values of 86.3 and 4.2 Å, respectively. | ||
| Fig. 4 The HR-TEM images (top) and SAED patterns (middle) of P1d (a) and HP1d (b), and a schematic illustration of the regular structure (c, the size indicated was obtained from the XRD results). | ||
The first d value (86.3 Å) for HP1d is nearly twice as large as the length of a PBI branch (47 Å), illustrating that there might be an interpenetrating network between PBI branches on neighboring PE backbones of HP1d (Fig. 4c), and it is smaller than that (107.7 Å) of P1d, indicating that the PBI branches in HP1d penetrated more tightly with each other. The second-order diffraction peak corresponded to the π–π stacking distance, presumably arising from the cofacial interaction of PBI branches. The original crystallinity of the PE backbone was possibly disturbed by the π–π stacking interaction between the PBI branches. Consequently, this strong π–π stacking behavior may be responsible for such a long ordered conformation. Simultaneously, the van der Waals interactions between the neighboring polymeric backbones in the second dimension16 may also be responsible for such a long ordered pattern.
The above effects suggest that the overlapping of PBI branches between polymeric backbones may have generated very dense planes of perfectly π-stacked PBI units with an ordered stacking period (Fig. 4c). In contrast, poly(1,6-heptadiyne) bearing the same PBI branches was amorphous, with a disordered architecture,13 likely due to its more rigid polyacetylene backbone with five-membered ring units and dense distribution of PBI branches along the polyacetylene backbone than that of HP1d, implying that either the flexibility of the polymer backbone or the density of the PBI branches play an important role in the polymer morphology. From the above-mentioned investigation, it could be concluded that there is enough space between the bulky PBI branches to permit the penetration of PBI branches with each other, yielding an ordered architecture from the simple structure of PE with bulky substituents.
To investigate the overlapping molecules in detail, information about the morphology of these polymers was obtained using AFM analysis via the tapping mode. A dilute solution of polymer at a concentration of 0.0025 mg mL−1 in chloroform was drop-coated onto mica for AFM imaging, and regularly assembled pillars stacked in a parallel manner with widths of 21–89 nm and heights of 1.3–1.6 nm were vividly observed (Fig. 5 and Fig. S5 (ESI†)). In addition, three-dimensional side views showed the nanostructured pillars grown nicely in directions perpendicular to the mica surface, and all of them were, in fact, made of numerous single filaments of varying lengths.
 | ||
| Fig. 5 AFM images of P1d (a) and HP1d (b) at 0.0025 mg mL−1. The left column shows 3D images and the right column shows 2D images. | ||
Spectroscopic and redox properties
PBI has been shown to be an excellent photofunctional building block for light harvesting and the most promising candidate for n-type semiconductor materials. Hence, UV-Vis, CV, and fluorescence analysis can provide additional information about the PBI-branched polymer structure and optical properties. Fig. 6a shows the characteristic absorption (400–600 nm) of the PBI branch. The PBI absorption maximum (λmax) at 523 nm with a strongly pronounced vibronic fine structure was observed in CHCl3 solution, and resulted from the electronic S0–S1 transition with a transition dipole moment along the molecular axis.17 A second absorption band emerged at lower wavelengths (400–460 nm), and was due to the electronic S0–S2 transition with a transition dipole moment perpendicular to the long molecular axis.18 The absorption at 230 nm was also due to the conjugated PBI branch, and the n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N groups appeared at 275 nm. The typical fluorescence change is shown in Fig. S6.† The dilute CHCl3 solution of 1 was observed to be highly emissive under UV illumination, whereas the fluorescence intensities of P1 and HP1 were relatively low. This behavior may have been induced by the enhanced π–π interaction between PBI mioeties of P1 and HP1. The fluorescence quantum yield (ΦF) of monomer 1 was 69%, while after ADMET polymerization and hydrogenation, the ΦF values of P1 and HP1 were less, at 51% and 53%, respectively (Fig. S6†). It is clear that the ΦF values of the polymers were lower than that of monomer 1, likely due to the enhanced π–π stacking behavior induced by the overlapping of PBI branches between neighboring polymeric backbones.
O and C–N groups appeared at 275 nm. The typical fluorescence change is shown in Fig. S6.† The dilute CHCl3 solution of 1 was observed to be highly emissive under UV illumination, whereas the fluorescence intensities of P1 and HP1 were relatively low. This behavior may have been induced by the enhanced π–π interaction between PBI mioeties of P1 and HP1. The fluorescence quantum yield (ΦF) of monomer 1 was 69%, while after ADMET polymerization and hydrogenation, the ΦF values of P1 and HP1 were less, at 51% and 53%, respectively (Fig. S6†). It is clear that the ΦF values of the polymers were lower than that of monomer 1, likely due to the enhanced π–π stacking behavior induced by the overlapping of PBI branches between neighboring polymeric backbones.
In order to gain insight into the intrinsic electrochemical properties of the PBI-based PE, the CV analysis was carried out in film (on a glassy carbon electrode). The material exhibited well-reversible oxidative and reductive processes. It displayed three successive reversible monoelectronic reduction waves around −1.12, −0.84, and −0.75 V (Fig. 6b), which are classically found for PBI. Using the reported method,17 the energy of the lowest unoccupied molecular orbital (LUMO) was obtained from the onset reduction potential (Eonsetred): ELUMO = −(4.4 + Eonsetred), and this LUMO energy of HP1d was calculated to be low, at −3.75 eV. Compared with the PBI derivatives of low molar mass (e.g., PDI-FCN2),19 HP1d was shown to have a higher LUMO level, which could offer the solar cell devices with a high open-circuit voltage.
Thermal properties
Thermal properties of the unsaturated P1 and saturated HP1 were systematically investigated by TGA and DSC techniques. The thermal decomposition temperatures (Td) at 5% weight loss of the representative polymers P1a, P1d, HP1a, and HP1d were determined, from their TGA curves (Fig. 7a, Table 1), to be 263, 367, 295, and 385 °C, respectively, indicating their highly thermal stability; these data are also similar to the Td values of previously reported PE polymers bearing periodically distributed single ethyl acrylate groups attached to PE carbons.20 The unsaturated polymers P1a and P1d decomposed completely at 780 °C, while 27% and 40% of the weights of the saturated polymers HP1a and HP1d, respectively, were still retained when they were heated to 800 °C; these latter amounts of residue are obviously greater than that of the hydrogenated ethylene–ethyl acrylate copolymers,21 and are indicative of the excellent thermal stability of PBI-containing PE.Glass transition and melting behaviors of the unsaturated P1 and saturated HP1 were characterized by DSC. As depicted in Fig. 7b, P1d exhibited an obvious glass transition according to the DSC curve, with a Tg value of 80.4 °C. For HP1d, the glass transition was detected at a Tg value of 75.8 °C, which is slightly lower than that of its precursor P1d, but beyond what was expected and much higher than those of PE polymers with well-defined short or long alkyl branches and even large pyrene branches,22,23 as well as of ADMET polyesters with amide branches.24 More importantly, the change in the Tg value that occurred upon saturation of P1d to form HP1d is also different from such changes of previously reported well-defined polyolefins, where the Tg of the saturated polymer increased slightly compared to the unsaturated analogue; these observations for polyolefins were indicative of the segmented motion of the branches being restricted by the crystallinity of the PE backbone.23c The shift of Tg to a much higher temperature relative to previous perfectly sequenced EP or EO copolymers with methyl or hexyl branches22,23 is attributed to the polymer chain flexibility being inhibited by the bulky rigid PBI branches, and the backbone crystallization being impeded by strong π–π stacking interactions and aggregation of PBI. However, the DSC thermograms for these polymers showed no melting transition up to 200 °C. PE is a typical (semi)crystalline polymer, while for this PE derivative, HP1d, the bulky PBI branches on every 21st carbon can disturb its otherwise inherently crystalline behavior. This interference was apparently due to the intermediate branch density in HP1d impeding crystallite formation even though the number of methylene groups between PBI branches was more than 15.23 Longer methylene spacers and weaker interactions of branches may reduce this type of disturbance. Additionally, the values of Tg were reduced to 76.5 and 59.2 °C for P1c and P1a, and 64.5 and 51.8 °C for HP1c and HP1a (Table 1), respectively, which was in agreement with the trend of molecular weight being decreased.
Conclusions
In summary, we have demonstrated a synthetic pathway to produce PE derivatives with PBI groups attached in a precisely periodically repeating manner, via ADMET polymerization and followed by exhaustive hydrogenation. This type of polymer product is of particular interest in optoelectronic applications since it differs from the previous metathesis-derived linear PE analogues not only in that it has a distribution of bulky branches precisely every 21st carbon, but also possesses a potentially useful building block of PBI, a rigid aryl chromophore, which is capable of electron transport as an n-type semiconductor. Examples of this PE derivative demonstrated good thermal stability with relatively high Tg values (51.8–75.8 °C), higher Td values (295–385 °C), and 27–40% residual weights even when they were heated to 800 °C, compared with those of other PEs. Also, this polymer displayed a wider range of light absorption (230–590 nm) and higher LUMO energy level (−3.75 eV), which could offer solar cell devices with a high open-circuit voltage. Although it exhibited no PE crystallinity, likely due to the overlapping of the PBI branches, the polymeric backbones generate a regular architecture consisting of periodic stacking. This feature makes it interesting for prospective applications in solar cells and other optoelectronic devices. The HP1 polymer combines the advantages of polyethylene and the optical properties of PBI. Further studies will be directed to explore the use of these new copolymers as functional materials for opto-electronic applications.Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 21374030, No. 21074036), and Large Instruments Open Foundation of East China Normal University (No. 2014–23) for financial support of this work.Notes and references
- S. D. Ittel, L. K. Johnson and M. Brookhart, Chem. Rev., 2000, 100, 1169–1204 CrossRef CAS PubMed.
- S. E. Lehman, K. B. Wagener, L. S. Baugh, S. P. Rucker, D. N. Schulz, M. Varma-Nair and E. Berluche, Macromolecules, 2007, 40, 2643–2656 CrossRef CAS.
- S. Song, W. Miao, Z. Wang, D. Gong and Z. R. Chen, Polymer, 2015, 64, 76–83 CrossRef CAS PubMed.
- (a) D. Thompson, R. Yamakado and K. B. Wagener, Macromol. Chem. Phys., 2014, 215, 1212–1217 CrossRef CAS PubMed; (b) G. Rojas and K. B. Wagener, Macromolecules, 2009, 42, 1934–1947 CrossRef CAS.
- B. S. Aitken, M. Lee, M. T. Hunley, H. W. Gibson and K. B. Wagener, Macromolecules, 2010, 43, 1699–1701 CrossRef CAS.
- S. Yagai, M. Usui, T. Seki, H. Murayama, Y. Kikkawa, S. Uemura, T. Karatsu, A. Kitamura, A. Asano and S. Seki, J. Am. Chem. Soc., 2012, 134, 7983–7994 CrossRef CAS PubMed.
- C. Li and H. Wonneberger, Adv. Mater., 2012, 24, 613–636 CrossRef CAS PubMed.
- S. Rajaram, R. Shivanna, S. K. Kandappa and K. Narayan, J. Phys. Chem. Lett., 2012, 3, 2405–2408 CrossRef CAS.
- (a) S. M. Lindner, S. Hüttner, A. Chiche, M. Thelakkat and G. Krausch, Angew. Chem., Int. Ed., 2006, 45, 3364–3368 CrossRef CAS PubMed; (b) S. Rajaram, P. B. Armstrong, B. J. Kim and J. M. Fréchet, Chem. Mater., 2009, 21, 1775–1777 CrossRef CAS; (c) Q. Zhang, A. Cirpan, T. P. Russell and T. Emrick, Macromolecules, 2009, 42, 1079–1082 CrossRef CAS; (d) J. Huang, Y. Wu, H. Fu, X. Zhan, J. Yao, S. Barlow and S. R. Marder, J. Phys. Chem. A, 2009, 113, 5039–5046 CrossRef CAS PubMed.
- W. Jiang, C. Xiao, L. Hao, Z. Wang, H. Ceymann, C. Lambert, S. D. Motta and F. Negri, Chem.–Eur. J., 2012, 18, 6764–6775 CrossRef CAS PubMed.
- C. B. Nielsen, D. Veldman, R. Martín-Rapún and R. A. J. Janssen, Macromolecules, 2008, 41, 1094–1103 CrossRef CAS.
- T. E. Hopkins and K. B. Wagener, Macromolecules, 2004, 37, 1180–1189 CrossRef CAS.
- W. Song, H. J. Han, J. H. Wu and M. R. Xie, Chem. Commun., 2014, 50, 12899–12902 RSC.
- M. R. Xie, H. J. Han, O. Y. Jin and C. X. Du, Acta Chimica Sinica, 2013, 71, 1441–1445 CrossRef CAS.
- S. L. Lee, N. T. Lin, W. C. Liao, C. h. Chen, H. C. Yang and T. Y. Luh, Chem.–Eur. J., 2009, 15, 11594–11600 CrossRef CAS PubMed.
- P. O. Schwartz, L. Biniek, E. Zaborova, B. Heinrich, M. Brinkmann, N. Leclerc and S. Mery, J. Am. Chem. Soc., 2014, 136, 5981–5992 CrossRef CAS PubMed.
- Y. Li, Y. Cao, J. Gao, D. Wang, G. Yu and A. J. Heeger, Synth. Met., 1999, 99, 243–248 CrossRef CAS.
- R. Gvishi, R. Reisfeld and Z. Burshtein, Chem. Phys. Lett., 1993, 213, 338–344 CrossRef CAS.
- B. A. Jones, A. Facchetti, M. R. Wasielewski and T. J. Marks, J. Am. Chem. Soc., 2007, 129, 15259–15278 CrossRef CAS PubMed.
- T. W. Baughman, J. C. Sworen and K. B. Wagener, Macromolecules, 2006, 39, 5028–5036 CrossRef CAS.
- Z. Li, L. L. Li, X. X. Deng, A. Lv, C. H. Wang, F. S. Du and Z. C. Li, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 2900–2909 CrossRef CAS PubMed.
- J. C. Sworen, J. A. Smith, K. B. Wagener, L. S. Baugh and S. P. Rucker, J. Am. Chem. Soc., 2003, 125, 2228–2240 CrossRef CAS PubMed.
- (a) J. C. Sworen, J. A. Smith, J. M. Berg and K. B. Wagener, J. Am. Chem. Soc., 2004, 126, 11238–11246 CrossRef CAS PubMed; (b) J. C. Sworen and K. B. Wagener, Macromolecules, 2007, 40, 4414–4423 CrossRef CAS; (c) E. B. Berda and K. B. Wagener, Macromolecules, 2008, 41, 5116–5122 CrossRef CAS.
- A. Sehlinger, L. M. de Espinosa and M. A. R. Meier, Macromol. Chem. Phys., 2013, 214, 2821–2828 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Scheme, IR, NMR and emission spectra, AFM and TEM images, and XRD patterns. See DOI: 10.1039/c5ra10049f |
| This journal is © The Royal Society of Chemistry 2015 |