Related magnetic properties of CoFe2O4 cobalt ferrite particles synthesised by the polyol method with NaBH4 and heat treatment: new micro and nanoscale structures†
Abstract
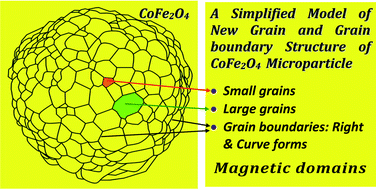
In this contribution, hierarchical CoFe2O4 particles are successfully prepared via a modified polyol elaboration method with NaBH4 and a proposed heat treatment process. Here, new as-prepared CoFe2O4 particles with sizes in the range of 5 μm show highly uniform characteristics in their size, shape and cubic spinel crystal structure according to X-ray diffraction (XRD), whole pattern fitting and Rietveld refinement, X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM). We discovered that the CoFe2O4 microparticles prepared in the certain size range of 5 μm show exciting configurations of grain and grain boundaries under particle heat treatment at the high temperature 900 °C. Finally, CoFe2O4 ferrite particles with various well-defined micro and nanoscale structures were produced after appropriate heat treatment processes under high temperature, and which have a high coercive field, HC, around 416–888 Oe, and the highest saturation magnetization, MS, of about 74–91 emu g−1 at room temperature (RT) for all of the as-prepared samples measured using a vibrating sample magnetometer (VSM). Here, the magnetic behavior has shown persuasive evidence that the desirable ferrimagnetic properties of CoFe2O4 oxides do not only depend on their size but also on the spinel structure of the CoFe2O4 oxides as well. Finally, the as-prepared CoFe2O4 particles with the formula CoO·Fe2O3 were regarded as the best inverse ferrimagnetic materials with magnetic parameters of HC at 896 Oe, MR/MS squareness around 0.420, and MS around 92 emu g−1 at 20 kOe for the downward part of the hysteresis loop.

 Please wait while we load your content...
Please wait while we load your content...