State of the art of biodiesel production processes: a review of the heterogeneous catalyst
Abstract
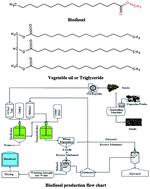
A broadened focus on energy, the fast growing value of petroleum oil, harmful atmospheric emissions because of the evolution of greenhouse gases, natural contamination, and quick reduction approaches to obtain fossil fuels are critical factors in the search for alternative energy sources. The need for developing renewable energy sources with fewer environmental effects is increasing because of the problems caused by the extensive use of fossil fuels. Currently, creating energy from low-carbon origins and introducing eco-friendly modern technology are the main targets of researchers in the field. Biodiesel has been identified as an alternative renewable liquid fuel source that can be derived through thermal cracking, esterification and transesterification of different triglycerides. Among these processes, the most popular and convenient technique for biodiesel production is the transesterification of triglyceride with the help of a suitable alcohol and a catalyst. Many scientists have introduced different types of catalysts to optimize the reaction conditions and the biodiesel production yields. Catalyst selection involves the determination of the water content and the free fatty acids in the oil. Homogeneous base catalysts provide faster reaction rates than homogeneous acid catalysts. Recently researchers have paid attention to heterogeneous catalysts because of their high activity, high selectivity, catalyst recovery, reusability, easy separation from the products, and water tolerance properties. Biocatalysts present significant advantages in terms of environmental issues over conventional alkali-catalyzed processes. This review article focuses on various technologies used for biodiesel production, as well as the benefits and limitations of the different types of catalysts in the relevant production technologies. We also conduct a comparative study of biocatalysts and homogeneous and heterogeneous catalysts in biodiesel production technologies at the laboratory scale, as well as their industrial applications.

 Please wait while we load your content...
Please wait while we load your content...