A versatile strategy for synthesis of hyperbranched polymers with commercially available methacrylate inimer†
Hongjun Yanga,
Tao Baia,
Xiaoqiang Xuea,
Wenyan Huanga,
Jianhai Chena,
Xiaolei Qiana,
Guangzhao Zhang*b and
Bibiao Jiang*a
aJiangsu Key Laboratory of Materials Surface Science and Technology, School of Materials Science and Engineering, Jiangsu Collaborative Innovation Center of Photovoltaic Science and Engineering, Changzhou University, Changzhou, Jiangsu, P. R. China 213164. E-mail: jiangbibiao@cczu.edu.cn
bFaculty of Materials Science and Engineering, South China University of Technology, Guangzhou, P. R. China 510640
First published on 1st July 2015
Abstract
Self-condensing vinyl polymerization (SCVP) provides an efficient approach for synthesis of hyperbranched polymers. However, most of the inimers employed for SCVP need to be synthesized before use. Here, we report a facile strategy to synthesize hyperbranched polymers under mild conditions by using a commercially available hydroxyl-substituted methacrylate as the inimer. The hyperbranched structures of the resulting polymers were confirmed by nuclear magnetic resonance, differential scanning calorimetry and size-exclusion chromatography equipped with online light scattering and viscosity detectors. The synthesis can be performed under mild reaction conditions. Particularly, this approach can be applied to not only the SCVP of vinyl monomers but also the self-condensing ring-opening polymerization of cyclic esters for preparation of hyperbranched polyesters. The present study provides a facile strategy to synthesize hyperbranched polymers.
Introduction
Hyperbranched polymers (HBPs) are three-dimensional polydisperse polymers with an elliptic randomly branched structure comprising linear units, branched units and terminal units.1–5 Compared with their linear analogs, HBPs possess unique physical and chemical properties such as higher solubility, lower viscosity, non-/low entanglement, and large numbers of terminal functional groups. Recent studies demonstrate that HBPs can self-assemble into various supramolecular structures such as nano or microscale vesicles, micelles, fibers, films and tubules.6–9 Thus, HBPs can find applications in drug delivery and other bio-related fields, which in turn motivates more intense efforts in their synthesis.9,10 The earlier synthesis of HBPs were often via the polycondensation of ABn-type monomers containing carboxyl, hydroxyl, and/or amine groups.11–15 Such a procedure cannot control the molecular weights and hyperbranched structures of the resulting polymers because the polymerization is uncontrolled. A chain walking polymerization strategy was developed later specifically for the synthesis of hyperbranched polyethylenes and functionalized polymers through ethylene coordination polymerization with the use of Brookhart Pd–diimine catalysts.16–20Since self-condensing vinyl polymerization (SCVP) using polyfunctional AB* monomers or the so-called “inimers” was first reported in 1995,21 it has extended to nitroxide-mediated radical polymerization, atom transfer radical polymerization,22–26 reversible addition–fragmentation chain transfer polymerization,27–30 group-transfer polymerization,31 and nitroxide polymerization.32–34 Besides the above controlled/living polymerization, SCVP was also applied to other monomers and polymerization procedures. For example, hyperbranched aliphatic polyesters were successfully synthesized by the self-condensing ring-opening polymerization (SCROP) of substituted cyclic esters.35–37 However, most of inimers used in SCVP or SCROP are not commercially available, need to be synthesized before use.38 For instance, the synthesis of a substituted lactone inimer for SCROP comprised four steps, and the product of each step required additional purification. It is still a challenge to use commercially available monomers as efficient inimers for the synthesis of HBPs by SCVP or SCROP.
In the present study, we report a facile one-pot synthesis of HBPs using a commercially available inimer, hydroxyethyl methacrylate (HEMA). This approach can be used in not only the SCVP of vinyl monomers but also the SCROP of cyclic esters, offering innovative insights into polymer synthesis. To our best knowledge, only a few reports about inimer using in two different types of polymerization.39,40
Experimental
Materials
ε-Caprolactone (CL) (from Aldrich) was dried over calcium hydride (CaH2) and distilled under reduced pressure prior to use. Methyl methacrylate (MMA) and HEMA (from Sinopharm) were distilled from CaH2 and stored under a nitrogen atmosphere at −20 °C. Benzyl alcohol (BA) (from Sinopharm) was distilled from CaH2 before use. (1-tert-butyl-4,4,4-tris(dimethylamino)-2,2-bis[tris(dimethylamino)phosphoranylidenamino]-2Λ5,4Λ5-catenadi(phosphazene)) (t-BuP4) was purchased from Aldrich and used as received. Tetrahydrofuran (THF) (from Sinopharm) was freshly distilled from sodium/benzophenone and stored under an argon atmosphere. Other reagents from Sinopharm were used as received.Characterization
The molecular weights and polydispersities of the samples were obtained by size-exclusion chromatography (SEC) equipped with online light scattering and viscosity detectors (TD-SEC) at 35 °C. The instrumentation consists of a Waters 1515 Isocratic HPLC pump with 5 mm Waters Styragel columns (guard, HR3, HR4, HR5, and HR6; the molecular weight ranges of the four HR columns are 500–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 5000–600
000, 5000–600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 50
000, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–4
000–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 200
000, and 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–10
000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, respectively), a Waters 717 PLUS Autosampler, a Waters 2414 Differential Refractive Index (DRI) detector with a wavelength of 880 nm, a multiangle laser light scattering (MALLS) detector (Wyatt mini-DAWN HELEOS-II) with an 18-angle light scattering detector at a wavelength of 690 nm and 220 W power, a Wyatt Visco Star viscometer detector, and a Waters Breeze data manager. The eluent was HPLC-grade THF with a flow rate of 1.0 mL min−1. The refractive index increments (dn/dC) determined using a Wyatt Optilab REX (λ = 640 nm) interferometric differential refractometer in batch mode at 35 °C for PCL and PMMA were 0.077 and 0.083 mL g−1, respectively.
000 g mol−1, respectively), a Waters 717 PLUS Autosampler, a Waters 2414 Differential Refractive Index (DRI) detector with a wavelength of 880 nm, a multiangle laser light scattering (MALLS) detector (Wyatt mini-DAWN HELEOS-II) with an 18-angle light scattering detector at a wavelength of 690 nm and 220 W power, a Wyatt Visco Star viscometer detector, and a Waters Breeze data manager. The eluent was HPLC-grade THF with a flow rate of 1.0 mL min−1. The refractive index increments (dn/dC) determined using a Wyatt Optilab REX (λ = 640 nm) interferometric differential refractometer in batch mode at 35 °C for PCL and PMMA were 0.077 and 0.083 mL g−1, respectively.
Nuclear magnetic resonance (NMR) spectra were recorded using a Bruker ARX400 NMR spectrometer, deuterated chloroform (CDCl3) as the solvent, and tetramethylsilane (TMS) as the internal standard.
Differential scanning calorimetry (DSC) was performed using a DSC Q2000 analyzer at a nitrogen flow rate of 50 mL min−1. Samples were quickly heated to 150 °C and kept for 10 min to remove thermal history, then cooled to −50 °C at a rate of 10 °C min−1. Finally, they were reheated to 150 °C at the same rate.
Synthesis of linear polymers using BA as the initiator
A typical polymerization was performed as follows: CL (0.50 g, 4.4 mmol, 150 equiv.), BA (3.10 μL, 0.030 mmol, 1.0 equiv.), and toluene (0.58 mL) were added to a flame-dried and nitrogen-purged round-bottom flask equipped with a magnetic stirrer. t-BuP4 (37.5 μL, 0.030 mmol, 1.0 equiv. in hexane) was added through a rubber septum by a syringe to start the polymerization at 90 °C. The polymerization was terminated with hydrochloric acid/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v). The product was dissolved in THF and precipitated in a large excess of hexane. After the filtration, the polymer was dried under vacuum. The polymerization of MMA initiated by BA was carried out using a similar procedure except that the polymerization temperature was 25 °C.
20, v/v). The product was dissolved in THF and precipitated in a large excess of hexane. After the filtration, the polymer was dried under vacuum. The polymerization of MMA initiated by BA was carried out using a similar procedure except that the polymerization temperature was 25 °C.
Synthesis of hyperbranched polymers using HEMA as the inimer
A typical polymerization was performed as follows: CL (0.20 g, 1.75 mmol, 20 equiv.), HEMA (10.6 μL, 0.0875 mmol, 1.0 equiv.), and toluene (0.23 mL) were added to a previously flamed and nitrogen-purged round-bottom flask equipped with a magnetic stirrer. The flask was placed in an oil bath at 90 °C. t-BuP4 (109 μL, 0.0875 mmol, 1.0 equiv. in hexane) was added through a rubber septum by a syringe to start the polymerization. After the polymerization for 8 h, the reaction mixture was poured into n-hexane. The precipitate was filtered, washed with n-hexane, and dried under vacuum at room temperature. The copolymerizations of MMA and HEMA were carried out using a similar procedure except that the polymerization temperature was 25 °C.Results and discussion
The aim of this study is to develop a relatively general method for the synthesis of HBPs from cyclic and/or vinyl monomers by using a commercially available inimer. Thus, we used CL and MMA as the typical cyclic and vinyl monomers, respectively.Synthesis of hyperbranched polyesters using a commercially available methacrylate inimer
Generally, only cyclic esters substituted with hydroxyl groups can be used as the inimers for SCROP. A hydroxyl-functionalized methacrylate such as HEMA can be used for vinyl polymerization, but not for the SCROP of cyclic esters because vinyl and cyclic monomers are quite different in the reactivity and polymerization mechanism. These monomers also cannot be directly employed for anionic polymerization since they react immediately with the initiators, or crosslinked were observed during the polymerization.41,42 Our earlier study reveals that t-BuP4 can catalyze the hybrid copolymerization of CL and MMA, which is a combination of vinyl polymerization and ring-opening polymerization (ROP).43,44 So, it is possible to dedicate that HEMA can be used as the inimer to copolymerize with CL to form hyperbranched polyester. We first examined the homopolymerization of HEMA with t-BuP4 as the catalyst, where the molar ratio of HEMA to t-BuP4 is 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 25 °C and 90 °C, respectively. Yet, crosslinking happens in the homopolymerizatin once t-BuP4 was introduced. The fact indicates that the hydroxyl in HEMA can act as an initiator of the vinyl polymerization in the presence of t-BuP4.
1 at 25 °C and 90 °C, respectively. Yet, crosslinking happens in the homopolymerizatin once t-BuP4 was introduced. The fact indicates that the hydroxyl in HEMA can act as an initiator of the vinyl polymerization in the presence of t-BuP4.
The copolymerization was carried out at 90 °C with a CL/HEMA molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Unlike the homopolymerization of HEMA, no crosslinking occurs in the copolymerization when CL/HEMA molar ratio is higher than 20/1. For comparison, we have also synthesized linear poly(ε-caprolactone) (l-PCL1) by using BA as the initiator of ROP of CL under the same conditions. Table 1 shows that SCROP of HEMA and CL yields a polymer (h-PCL1) with an average-number molecular weight (Mn.SEC) of 1.13 × 104 g mol−1, about ten times higher than that of l-PCL1. Fig. 1 shows the SEC curves for the resulting polymers measured by a DRI detector. Clearly, l-PCL1 has a narrow distribution. In contrast, h-PCL1 shows a broad distribution. The molecular weight of h-PCL1 determined by light scattering (Mw.MALLS) is higher than that measured by DRI (Mw.SEC). (Table 1 and Fig. S1 in the ESI†). Moreover, the line for the elution time dependence of molecular weight of h-PCL1 lied significantly above the corresponding line of l-PCL1 (see Fig. S2 in the ESI†). The facts indicate that h-PCL1 has a hyperbranched structure.45,46
1. Unlike the homopolymerization of HEMA, no crosslinking occurs in the copolymerization when CL/HEMA molar ratio is higher than 20/1. For comparison, we have also synthesized linear poly(ε-caprolactone) (l-PCL1) by using BA as the initiator of ROP of CL under the same conditions. Table 1 shows that SCROP of HEMA and CL yields a polymer (h-PCL1) with an average-number molecular weight (Mn.SEC) of 1.13 × 104 g mol−1, about ten times higher than that of l-PCL1. Fig. 1 shows the SEC curves for the resulting polymers measured by a DRI detector. Clearly, l-PCL1 has a narrow distribution. In contrast, h-PCL1 shows a broad distribution. The molecular weight of h-PCL1 determined by light scattering (Mw.MALLS) is higher than that measured by DRI (Mw.SEC). (Table 1 and Fig. S1 in the ESI†). Moreover, the line for the elution time dependence of molecular weight of h-PCL1 lied significantly above the corresponding line of l-PCL1 (see Fig. S2 in the ESI†). The facts indicate that h-PCL1 has a hyperbranched structure.45,46
| Sample | XHb | CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XH XH |
Conv.CL (%) | Mw.MALLSd (104 g mol−1) | Mn.SECc (104 g mol−1) | PDIc (%) | [η]e (mL g−1) | Rhe (nm) | g′f | Tg (°C) | Xc (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Polymerized at 90 °C for 8 h.b Initiator or inimer.c Determined with a DRI detector.d Determined with a MALLS detector.e Determined with a viscosity detector.f g′ = ([η])branched/([η])linear. | |||||||||||
| l-PCL1 | BA | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.5 | 0.15 | 1.50 | 0.72 | |||||
| l-PCL2 | BA | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97.9 | 1.11 | 1.13 | 1.43 | 25.6 | 4.2 | −65.2 | 61.1 | |
| h-PCL1 | HEMA | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.7 | 1.13 | 4.65 | 3.39 | 29.5 | 5.5 | 0.44 | −55.6 | 41.5 |
| h-PCL2 | HEMA | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.6 | 1.01 | 2.75 | 2.31 | 24.9 | 3.8 | 0.66 | −62.6 | 56.5 |
| h-PCL3 | HEMA | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.3 | 1.29 | 2.47 | 2.10 | 27.4 | 9.5 | 0.73 | −60.9 | 58.2 |
Degree of branching (DB) is an important characteristic parameter of branched polymers. Generally, DB, cannot be directly determined by spectroscopy. Fortunately, HBPs exhibit very different solution properties than their linear analogs, providing an insight for analyzing and characterizing hyperbranched structures.47,48 The hydrodynamic radius (Rh) and intrinsic viscosity ([η]) can be readily determined by using MALLS and a viscometer, respectively.49–51 The values of Rh and [η] for h-PCL1 is 5.5 nm and 29.5 mL g−1, respectively. Rh of l-PCL1 cannot be detected due to its low molecular weight. For comparison, we synthesized linear PCL (l-PCL2) with a relatively high molecular weight. Fig. 2a shows the molecular weight (Mw) dependence of Rh. h-PCL1 has much smaller Rh than l-PCL2 with the same Mw. This is another evidence that h-PCL1 has hyperbranched structure. The slope (0.46) for h-PCL1 is lower than that (0.57) for l-PCL2, further indicating the hyperbranched structure of h-PCL.52
 | ||
| Fig. 2 Molecular weight (Mw) dependence of (a) the hydrodynamic radius (Rh) and (b) intrinsic viscosity ([η]) for h-PCL1 and l-PCL2. | ||
Fig. 2b shows that h-PCL has lower viscosity than l-PCL at the same molecular weight. Moreover, the Mark–Houwink exponent m (0.41) for h-PCL1 is much lower than that (0.71) for l-PCL2. The facts clearly indicate the branched nature of h-PCL1. The Zimm branching factor g′ of polymers can be estimated by using the following equation:51,53
| g′ = ([η])branched/([η])linear |
Fig. 3 shows the DSC curves for l-PCL2 and h-PCL1. The enthalpy change (ΔHm) of melting for each sample is listed in Table 1. h-PCL1 shows melting with two peaks at ∼33.0 °C and ∼42.5 °C, respectively. Both melting temperatures are lower than that for l-PCL2 (∼53.1). The degree of crystallinity can be estimated from the enthalpy change divided by that for 100% crystalline PCL (ΔH0m = 136.4 J g−1).55 The degree of crystallinity for h-PCL1 is 41.6%, much lower than that for l-PCL2 (61.1%). This is because the branching in h-PCL1restricts the packing and folding of segments and thus reduces the chain crystallization.56
The effect of [CL]/[HEMA] molar ratio on the polymerization was studied at 90 °C. Table 1 shows the molecular weight and the polydispersity of the resulted polymer. Clearly, each h-PCL sample has a high molecular weight and a broad distribution, implying it has a branched structure. The Zimm branching factor g′ for each h-PCL is less than one, and g′ increases in the order h-PCL3 < h-PCL2 < h-PCL1, indicating the DB increases as [CL]/[HEMA] molar ratio decreases. This is understandable because the branching species increases as [CL]/[HEMA] decreases.
Based on the above results, we propose a mechanism about the formation of hyperbranched polyesters (Scheme 1). In the initiation step, t-BuP4 deprotonates the hydroxyl group in HEMA generating methacrylate anions. t-BuP4 may also react with the more hydrophobic vinyl group, but it is much slower than the reaction between t-BuP4 and hydroxyl. The formed anions react with CL, yielding a linear polymer with methacrylate groups at the terminal positions. Simultaneously, the formed anions can also react with the methacrylate groups in the polymer chains, yielding a branching site that can subsequently propagate to generate a HBP.
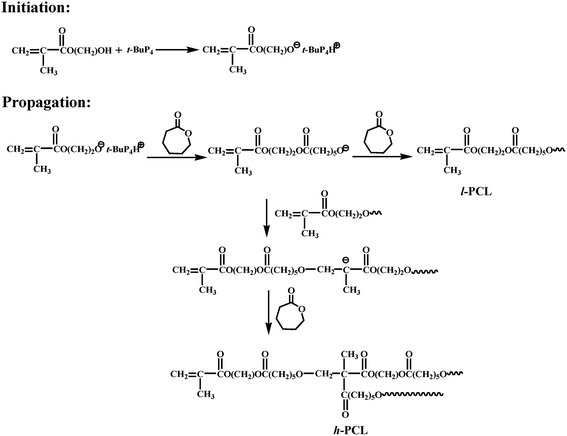 | ||
| Scheme 1 Proposed mechanism for the synthesis of h-PCL using a commercially available methacrylate inimer. | ||
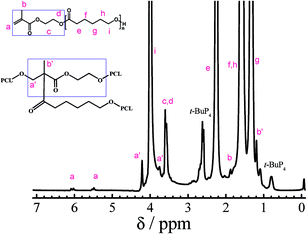
The mechanism is supported by 1H-NMR. As shown in Fig. 4, the signals of the reacted methacrylate groups assigned to peaks a′ and b′ appear at δ 4.21 and 1.18 ppm, whereas the unreacted methacrylate groups corresponding to peaks a and b appears at δ 6.03, 5.80, and 1.88 ppm. Note that the unreacted methacrylate groups provide reactive sites for the subsequent chemical modification of the polymer.
Synthesis of hyperbranched PMMA using a commercially available methacrylate inimer
HEMA serve as an efficient inimer not only for ROP of CL but also for the synthesis of hyperbranched poly(methyl methacrylate) (h-PMMA). Unlike SCVP of CL and HEMA, the polymerization of MMA and HEMA usually results in crosslinking, particularly at a high reaction temperature and low monomer concentration. To avoid the cross-linking, the polymerization was performed at 25 °C with an MMA/HEMA molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The solvent (toluene) was twice of the monomer in volume. Linear poly(methyl methacrylate) (l-PMMA) samples were also prepared using BA as the initiator with an MMA/BA molar ratio of 20
1. The solvent (toluene) was twice of the monomer in volume. Linear poly(methyl methacrylate) (l-PMMA) samples were also prepared using BA as the initiator with an MMA/BA molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 100
1 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The molecular weights of the polymers are listed in Table 2.
1. The molecular weights of the polymers are listed in Table 2.
| Sample | XHb | MMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XH XH |
Conv.MMA (%) | Mn.SECc (104 g mol−1) | Mw.MALLSd (104 g mol−1) | PDIc (%) | [η]e (mL g−1) | Rhe (nm) | g′f | Tg (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| a Polymerized at 25 °C for 8 h.b Initiator or inimer.c Determined with a DRI detector.d Determined with a MALLS detector.e Determined with a viscosity detector.f g′ = ([η])branched/([η])linear. | ||||||||||
| l-PMMA1 | BA | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.8 | 0.27 | 0.73 | 1.39 | 0.18 | 61.9 | ||
| l-PMMA2 | BA | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.2 | 2.81 | 4.10 | 1.24 | 30.5 | 7.1 | 108.8 | |
| h-PMMA1 | HEMA | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.4 | 0.38 | 16.1 | 3.89 | 28.4 | 13.7 | 0.24 | 101.3 |
| h-PMMA2 | HEMA | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.6 | 1.13 | 26.9 | 3.44 | 13.4 | 7.7 | 0.28 | 102.3 |
| h-PMMA3 | HEMA | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99.2 | 1.25 | 15.4 | 2.74 | 22.6 | 5.3 | 0.82 | 106.1 |
Fig. 5 shows SEC curves for PMMAs. DRI data show l-PMMA1 has unimodal and symmetrical distribution, whereas h-PMMA1 has a broader and asymmetrical distribution with higher molecular weight. MALLS data show that h-PMMA1 synthesized using HEMA as the inimer has significantly higher molecular weight than that for l-PMMA1 synthesized under the same reaction conditions. Also, the molecular weight detected by MALLS is significantly higher than that detected by DRI (Table 2). All the facts indicate a hyperbranched structure of h-PMMA1. Moreover, h-PMMA1 always has a larger Mw than h-PMMA1 at any given elution volume (Fig. 6S in the ESI†), further indicating a hyperbranched structure of h-PMMA1.
Fig. 6 shows the molecular weight dependence of hydrodynamic radius of PMMA samples. For the same Mw, h-PMMA1 always has a lower Rh lower than l-PMMA2, clearly indicating that h-PMMA1 has a hyperbranched structure. On the other hand, h-PMMA1 always has a lower viscosity [η] than l-PMMA2 with the same Mw, further indicating the hyperbranched structure of h-PMMA1. The Mark–Houwink exponent α (0.63) for h-PMMA1 is lower than that (0.69) for l-PMMA2. The average Zimm branching factor g′ (0.24) for h-PMMA1 is much less 1. All the facts demonstrate that h-PMMA1 has a hyperbranched structure. Moreover, g′ decreases from 0.32 to 0.20 as molecular weight increases, indicating that all the h-PMMA1 fractions have hyperbranched structures, and the branching level increases with molecular weight (see Fig. 7S in the ESI†).
 | ||
| Fig. 6 Molecular weight (Mw) dependence of (a) the hydrodynamic radius (Rh) and (b) intrinsic viscosity for h-PMMA1 and l-PMMA2. | ||
1H-NMR provides molecular evidences that h-PMMA1 is hyperbranched. Fig. 7 shows a series of broad peaks in the region 0.80–2.20 ppm attributed to the protons in the polymer backbone, and no peaks associated with the double bond of methacrylate appears at 5.78–6.03 ppm, indicating that all the vinyl groups are polymerized. The broad peaks located at 3.90–4.21 ppm attributed to the protons of the ether groups in HEMA units confirm the hyperbranched structures of h-PMMA1.
Fig. 8 shows DSC curves of PMMA samples. h-PMMA1 has a Tg much lower than l-PMMA2. This is because the branched polymers have more chain ends and the segments are more mobile, so that they have larger free volume and a lower Tg.57 On the other hand, the Zimm branching factor g′ for h-PMMA decreases from 0.43 to 0.63 in the order of h-PMMA1 < h-PMMA2 < h-PMMA3, indicating the DB increases as [MMA]/[HEMA] molar ratio decreases.
Based on the above discussion, we propose the mechanism for the formation of hyperbranched PMMA (Scheme 2). In short, the OH group in HEMA is first deprotonated by t-BuP4, resulting in an active center with vinyl groups at the terminal positions. The active center further reacts with MMA, forming a linear PMMA. Moreover, the active center also reacts with the methacrylate groups of the HEMA segment, leading to a hyperbranched PMMA.
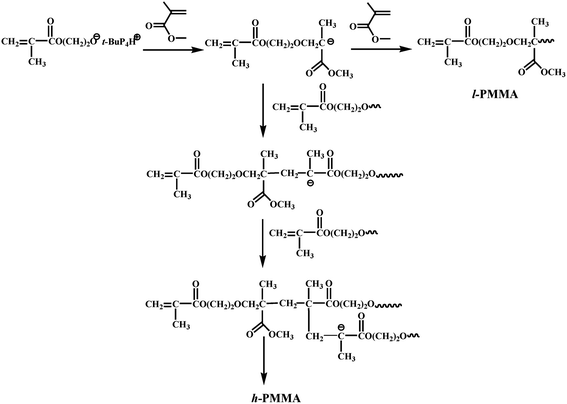 | ||
| Scheme 2 Proposed mechanism for the synthesis of h-PMMA using a commercially available methacrylate inimer. | ||
Conclusions
In conclusion, HBPs can be synthesized by using a commercially available hydroxyl-substituted methacrylate as the inimer. The hyperbranched structure can be confirmed by TD-SEC, NMR and DSC analyses. The approach can be used not only in the SCVP of vinyl monomers but also in the SCROP of cyclic esters to yield degradable hyperbranched polyesters. The present study provides a facile and versatile approach to synthesize hyperbranched polymers.Acknowledgements
The financial supports of this work by the National Natural Science Foundation of China (no. 21304010), the Natural Science Foundation of Jiangsu Province (BK20130246), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Program of Innovative Research Team of Changzhou University (ZMF13020026).References
- R. M. England and S. Rimmer, Polym. Chem., 2010, 1, 1533–1544 RSC.
- M. Jikei and M.-A. Kakimoto, Prog. Polym. Sci., 2001, 26, 1233–1285 CrossRef CAS.
- Y. Liu, C. Yu, H. Jin, B. Jiang, X. Zhu, Y. Zhou, Z. Lu and D. Yan, J. Am. Chem. Soc., 2013, 135, 4765–4770 CrossRef CAS PubMed.
- E. Sato, I. Uehara, H. Horibe and A. Matsumoto, Macromolecules, 2014, 47, 937–943 CrossRef CAS.
- W. Jiang, Y. Zhou and D. Yan, Chem. Soc. Rev., 2015, 44, 3874–3889 RSC.
- F. Sun, X. Luo, L. Kang, X. Peng and C. Lu, Polym. Chem., 2015, 6, 1214–1225 RSC.
- D. Yan, Y. Zhou and J. Hou, Science, 2004, 303, 65–67 CrossRef CAS PubMed.
- Y. Zhou and D. Yan, Chem. Commun., 2009, 1172–1188 RSC.
- F. L. Hatton, P. Chambon, T. O. McDonald, A. Owen and S. P. Rannard, Chem. Sci., 2014, 5, 1844–1853 RSC.
- T. Zhao, Y. Zheng, J. Poly and W. Wang, Nat. Commun., 2013, 4, 1873 CrossRef PubMed.
- R. Spindler and J. M. Frechet, Macromolecules, 1993, 26, 4809–4813 CrossRef CAS.
- Z. Xue, A. D. Finke and J. S. Moore, Macromolecules, 2010, 43, 9277–9282 CrossRef CAS.
- A. H. Müller, D. Yan and M. Wulkow, Macromolecules, 1997, 30, 7015–7023 CrossRef.
- D. Yan, A. H. Müller and K. Matyjaszewski, Macromolecules, 1997, 30, 7024–7033 CrossRef CAS.
- Y. Ohta, Y. Kamijyo, A. Yokoyama and T. Yokozawa, Polymers, 2012, 4, 1170–1182 CrossRef PubMed.
- Z. Guan, P. Cotts, E. McCord and S. McLain, Science, 1999, 283, 2059–2062 CrossRef CAS.
- Z. Ye, L. Xu, Z. Dong and P. Xiang, Chem. Commun., 2013, 49, 6235–6255 RSC.
- Z. Dong and Z. Ye, Polym. Chem., 2012, 3, 286–301 RSC.
- L. Xu and Z. Ye, Chem. Commun., 2013, 49, 8800–8802 RSC.
- Z. Ye and S. Li, Macromol. React. Eng., 2010, 4, 319–332 CrossRef CAS PubMed.
- C. J. Hawker, J. M. Frechet, R. B. Grubbs and J. Dao, J. Am. Chem. Soc., 1995, 117, 10763–10764 CrossRef CAS.
- S. G. Gaynor, S. Edelman and K. Matyjaszewski, Macromolecules, 1996, 29, 1079–1081 CrossRef CAS.
- H. J. Yang, B. B. Jiang, W. Y. Huang, D. L. Zhang, L. Z. Kong, J. H. Chen, C. L. Liu, F. H. Gong, Q. Yu and Y. Yang, Macromolecules, 2009, 42, 5976–5982 CrossRef CAS.
- H. Mori, A. Walther, X. Andre, M. G. LanzendZhang and L. Z. Konger, Macromolecules, 2004, 37, 2054–2066 CrossRef CAS.
- N. V. Tsarevsky, B. S. Sumerlin and K. Matyjaszewski, Macromolecules, 2005, 38, 3558–3561 CrossRef CAS.
- K. Min and H. Gao, J. Am. Chem. Soc., 2012, 134, 15680–15683 CrossRef CAS PubMed.
- Z. Wang, J. He, Y. Tao, L. Yang, H. Jiang and Y. Yang, Macromolecules, 2003, 36, 7446–7452 CrossRef CAS.
- A. Postma, T. P. Davis, G. Moad and M. S. O'Shea, Macromolecules, 2005, 38, 5371–5374 CrossRef CAS.
- S. Perrier, P. Takolpuckdee and C. A. Mars, Macromolecules, 2005, 38, 2033–2036 CrossRef CAS.
- S. G. Roy and P. De, Polym. Chem., 2014, 5, 6365–6378 RSC.
- P. F. Simon, W. Radke and A. H. Müller, Macromol. Rapid Commun., 1997, 18, 865–873 CrossRef CAS PubMed.
- Y. Tao, J. He, Z. Wang, J. Pan, H. Jiang, S. Chen and Y. Yang, Macromolecules, 2001, 34, 4742–4748 CrossRef CAS.
- A. Niu, C. Li, Y. Zhao, J. He, Y. Yang and C. Wu, Macromolecules, 2001, 34, 460–464 CrossRef CAS.
- C. Li, J. He, L. Li, J. Cao and Y. Yang, Macromolecules, 1999, 32, 7012–7014 CrossRef CAS.
- H. Yang, J. Xu, S. Pispas and G. Zhang, RSC Adv., 2013, 3, 6853–6858 RSC.
- J. Liu, W. Huang, Y. Zhou and D. Yan, Macromolecules, 2009, 42, 4394–4399 CrossRef CAS.
- M. Trollsås, P. Löwenhielm, V. Lee, M. Möller, R. Miller and J. Hedrick, Macromolecules, 1999, 32, 9062–9066 CrossRef.
- Z. Wei, X. Hao, P. A. Kambouris, Z. Gan and T. C. Hughes, Polymer, 2012, 53, 1429–1436 CrossRef CAS PubMed.
- X. H. Liu, Z. M. Dong, X. L. Tang and Y. S. Li, Polymer, 2010, 51, 854–859 CrossRef CAS PubMed.
- X. H. Liu, Y. M. Bao, X. L. Tang and Y. S. Li, Polymer, 2010, 51, 2857–2863 CrossRef CAS PubMed.
- B. A. Rozenberg, Polym. Bull., 2007, 58, 127–138 CrossRef CAS PubMed.
- A. Hirao, H. Kato, K. Yamaguchi and S. Nakahama, Macromolecules, 1986, 19, 1294–1299 CrossRef CAS.
- H. Yang, J. Xu and G. Zhang, Sci. China: Chem., 2013, 56, 1101–1104 CrossRef CAS.
- H. Yang, J. Xu, S. Pispas and G. Zhang, Macromolecules, 2012, 45, 3312–3317 CrossRef CAS.
- R. Baudry and D. Sherrington, Macromolecules, 2006, 39, 5230–5237 CrossRef CAS.
- M. A. Gonçalves, V. D. Pinto, R. Dias and M. R. P. Costa, Macromol. Symp., 2010, 296, 210–228 CrossRef PubMed.
- D. Konkolewicz, A. A. Gray-Weale and R. G. Gilbert, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 3112–3115 CrossRef CAS PubMed.
- Y. Zheng, W. Turner, M. Zong, D. J. Irvine, S. M. Howdle and K. J. Thurecht, Macromolecules, 2011, 44, 1347–1354 CrossRef CAS.
- M. Jikei, M. Suzuki, K. Itoh, K. Matsumoto, Y. Saito and S. Kawaguchi, Macromolecules, 2012, 45, 8237–8244 CrossRef CAS.
- R. K. Kainthan, E. B. Muliawan, S. G. Hatzikiriakos and D. E. Brooks, Macromolecules, 2006, 39, 7708–7717 CrossRef CAS.
- W. J. Wang, S. Kharchenko, K. Migler and S. Zhu, Polymer, 2004, 45, 6495–6505 CrossRef CAS PubMed.
- M. Chisholm, N. Hudson, N. Kirtley, F. Vilela and D. C. Sherrington, Macromolecules, 2009, 42, 7745–7752 CrossRef CAS.
- G. Saunders, P. A. Cormack, S. Graham and D. C. Sherrington, Macromolecules, 2005, 38, 6418–6422 CrossRef CAS.
- Z. M. Dong, X. H. Liu and Y. S. Li, Chin. J. Polym. Sci., 2009, 27, 285–291 CrossRef CAS.
- V. Crescenzi, G. Manzini, G. Calzolari and C. Borri, Eur. Polym. J., 1972, 8, 449–463 CrossRef CAS.
- H. Magnusson, E. Malmström, A. Hult and M. Johansson, Polymer, 2002, 43, 301–306 CrossRef CAS.
- A. Khalyavina, L. Häußler and A. Lederer, Polymer, 2012, 53, 1049–1053 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09851c |
| This journal is © The Royal Society of Chemistry 2015 |