TNPs as a novel fluorescent sensor for the selective recognition of fast green FCF: a spectrofluorimetric approach†
Dattatray K.
Dalavi
,
Avinash
Kamble
,
Dhanaji P.
Bhopate
,
Prasad G.
Mahajan
,
Govind B.
Kolekar
and
Shivajirao R.
Patil
*
Fluorescence Spectroscopy Laboratory, Department of Chemistry, Shivaji University Kolhapur, Maharashtra 416004, India. E-mail: srp_fsl@rediffmail.com; Fax: +91 2312692333; Tel: +91 9850092720
First published on 21st July 2015
Abstract
Fluorescent tetracene nanoparticles (TNPs) have been prepared by a reprecipitation method using cetyl trimethyl ammonium bromide (CTAB) as a stabilizer. These TNPs are more photostable against photobleaching and have a high solubility in water, minimizing the utilization of hazardous organic solvents when compared with single organic fluorophores in sensing applications. The method is based on the fluorescence quenching of the TNPs, which are used as a novel fluorescent sensor for the selective recognition of the fast green FCF dye in aqueous solution. The fluorescence intensity of the TNPs was quenched by the successive addition of increasing concentrations of the fast green FCF dye. The fluorescence quenching results were found to fit the Stern–Volmer (S–V) relationship in the range of 0.5–7.0 µg L−1 with a correlation coefficient of 0.999. The limit of detection (LOD) was 0.136 µg L−1. Moreover, the excited state lifetime of the TNPs remains unchanged even after increasing the concentration of the fast green FCF dye, which suggests that the fast green FCF dye was adsorbed over the surface of the nanoparticles to form a non-fluorescent ground state complex i.e. the nature of the quenching process is static. The proposed method was successfully applied for the quantitative analysis of the fast green FCF dye in commercial samples with no necessity of prior separation of analyte molecules from the interfering constituents.
1. Introduction
Dyes are the most visible sign of water pollution and they are hazardous to health because of the toxicity of their degradation products.1,2 Synthetic dyes are widely used in the textile, food, leather, cosmetics, paper, plastic and biomedical industries.3 The effluents of these industries dispose of huge amounts of dye contents which are mixed with water, which cause adverse effects on marine and human life. The available reports indicate that the textile manufacturers generate about 3.84 × 105 tons of waste each year.4Fast green FCF (Scheme 1) is a sea green triarylmethane synthetic food dye, also known as Food Green 3, FD and C Green no. 3. It can be used for tinned green peas and other vegetables, jellies, sauces, fish, desserts, and for dry bakery mix at levels of up to 100 mg kg−1. Though for a long time fast green FCF was in use as a colorant, the European Union and many other countries have banned its use for edible purposes due to its toxicity.5
Toxicological records reveal that fast green FCF is hazardous and allergenic for humans and animals. The tumorigenic effects of the dye in experiments on animals as well as the mutagenic effects in both experiments on animals and on humans have also been explored. Furthermore, it may cause eye and skin irritation and irritation in the upper respiratory tract in its undiluted form. It also acts as a presynaptic locus by inhibiting the release of neurotransmitters in the human/animal nervous system. Its carcinogenetic properties can produce sarcomas at the site of repeated subcutaneous injection.3–5 Hence a method of detection of fast green FCF in food is of significance in respect of the FDR and Environmental Pollution acts. The electro and spectrochromatographic methods reported for the determination of color additives in food products such as high performance liquid chromatography (HPLC) with photodiode array detection,6,7 HPLC with UV detection,8 HPLC-diode array detection,9 green chromatography,10 HPLC-diode array detector-electrospray mass spectrometry,11 capillary electrophoresis spectrophotometric detection,12 HPLC with UV-DAD detection,13 LC-UV detection,14 LC-electrospray mass spectrometry,15 HPLC and TLC-spectrophotometry,16 TLC and ion-pair HPLC,17 CPE-scanometry3 and photocolorimeter methods18 all involve more complicated and prolonged procedures, which are not suitable for U.S. Food and Drug Administration use. However the optical methods based on absorption and fluorescence are more simple and less expensive.
The general requirements for newly developed analytical methods are not only selectivity and sensitivity but also, the method needs to be green.10 Most of the organic syntheses and/or designs of nanoparticles, nanofibers, etc. use highly volatile organic solvents which increase the risk of fire, explosion and water contamination. These organic solvents can also act as air pollutants causing ozone depletion, photochemical smog and global warming.19
The aqueous suspension of fluorescent organic nanoparticles prepared by a modified reprecipitation20 method minimized the use of organic solvents and was more efficient in sensitivity due to the Aggregation Induced Enhanced Emission (AIEE). The nanoparticle suspension has a high colloidal stability, less photobleaching and absorption specificity when compared to a micellar solution of small fluorescent molecules. The aqueous suspension of TNPs with its surface intentionally modified to have positive zeta potential by using cetyltrimethyl ammonium bromide (CTAB) was found to exhibit affinity towards the fast green FCF dye when examined by a fluorescence quenching technique. The present paper reports the preparation of highly stable intensely fluorescent TNPs by minimal utilization of organic solvents and toxic reagents for the selective sensing of the dye without any interference from coexisting anions.
2. Experimental
2.1 Reagents
All chemicals used are of analytical reagent grade and were used as received without further purification. The required aqueous solutions were prepared with doubly distilled water. Tetracene was procured from Sigma Aldrich. Cetyl trimethyl ammonium bromide (CTAB) and fast green FCF were procured from Spectrochem Pvt. Ltd Mumbai and Loba Chemie, Mumbai (India) respectively.2.2 Instrumentation
The absorption spectra were recorded on a UV-VIS-NIR spectrophotometer using a 1.0 cm quartz cell at room temperature on Shimadzu UV-3600. Fluorescence measurements of the nanoparticle suspension were done with a PC based spectrofluorophotometer (JASCO Model FP-8300, Japan). Both excitation and emission slits were fixed at 10 nm. The excitation wavelength of 318 nm and the emission wavelength of 533 nm of the aqueous suspension was obtained from the excitation and the emission spectrum respectively. The particle size distribution and zeta potential of the TNPs in the aqueous suspension was measured by Dynamic Light Scattering (DLS) with a Zeta Sizer Nano ZS (Malvern) Instruments Ltd, UK. A Field Emission Scanning Electron Microscope (FESEM) Mira3 XM, Tescan, was used to examine the morphology and size of the nanoparticles. The fluorescence lifetimes in the time scan of 500 ps to 1 µs were measured by the Time Correlated Single Photon Counting (TCSPC) method of Horiba Sci. NL (Japan) at the approximate emission and excitation wavelengths of 533 nm and 320 nm respectively.2.3 Preparation of the CTAB stabilized TNPs
The water soluble TNPs stabilized by CTAB were prepared by the reprecipitation method with some essential modifications.20–23 1 ml of a tetracene solution in THF (0.1 mM) was injected by a microsyringe into 99 ml aqueous solution of CTAB (0.05 mM) with vigorous stirring. The role of the CTAB is to stabilize the growth of the tetracene nanoparticles and also to generate a positive surface charge. The mixture was sonicated for 20 min at 301 K to form stable nanoparticles of nanofiber shaped morphology.3. Results and discussion
3.1 Size distribution and morphology of the TNPs
The cationic surfactant CTAB, used as an additive, was found to control not only the size and shape of the organic nanomaterials, but also induced the charge on the surface of nanomaterials required for the electrostatic attraction of the fast green FCF dye. The size distribution histograms of the TNPs in an aqueous suspension prepared with and without CTAB, recorded by the DLS technique, are presented in Fig. 1. The histogram (Fig. 1a) reveals the size distribution of the TNPs in an aqueous suspension in the absence of the surfactant in a range of 68.06 nm to 712.4 nm. The maximum mean is 24.3% and the average particle size is about 122 nm. On the contrary the histogram in Fig. 1b reveals the narrow size distribution of the TNPs in the presence of the CTAB surfactant in a range of 8.72 nm to 15.69 nm. The maximum mean is 36.2% and the average size is about 12.2 nm. | ||
| Fig. 1 (a) Size distribution histograms of the TNPs in an aqueous suspension in the absence of the surfactant and (b) in the presence of the CTAB surfactant. | ||
The significance of the zeta potential is that its value can be related to the stability of the colloidal dispersions. The surface electrical zeta (ζ) potential measurement was taken to characterize the surface charge on the TNPs and to examine the stability. The zeta potential of the CTAB stabilized TNPs is 28.3 mV and that of the TNPs without surfactants is −3.2 mV. The value of the zeta potential in the range from −25 mV to +25 mV is an indication of the higher level stability of the nanoparticle suspension.24,25 The low potential tends to exceed the attraction between colloids and their repulsion breaks the dispersion. Consequently, colloids with low zeta potentials tend to coagulate or flocculate.20 As a result, the modified reprecipitation method was developed to design stable fluorescent nanoparticles in the presence of the CTAB surfactant. The high-resolution, three-dimensional images produced by the field emission scanning electron microscopes (FESEMs) provide topographical, morphological and compositional information which makes them valuable in a variety of scientific and industrial applications. The FESEM photograph of the air-dried films of the TNPs in Fig. 2 reveals that the aggregated particles are in the form of nanofibers. For the FESEM the mass-weighted particle size distribution suffers from agglomeration problems of the nanoparticles due to the drying phase in sample preparation.25,26
3.2 Photophysical properties of the CTAB stabilized TNPs
Aggregation Induced Enhanced Emission (AIEE) and absorption properties of the TNPs examined by spectroscopy studies are to be used as probe for sensing applications. Fig. 3 displays the UV-vis absorption spectra of the TNPs in an aqueous dispersion and in the dilute solution of tetracene in THF. The observation of the spectra in the figure reveals that the spectrum of the dilute solution has five clearly resolved peaks at 375, 396, 418, 443 and 473 nm. Moreover, no spectral shift was observed when the concentration of the dilute solution was increased from 0.5 × 10−5 to 0.5 × 10−6 mol L−1. In contrast the absorption peak of the TNPs is red shifted with a broad peak width. The absorption maximum appearing at 533 nm shows a spectral shift of about 166![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cm−1 from the absorption peak of the tetracene monomer appearing at 473 nm. The results suggest that J-aggregation occurs, where the molecules arrange into a slanted stack and the transition to the lower couple excited state of molecules is allowed. Accordingly, the absorption is red-shifted and intensely narrow. The molecules in the nanoparticles may be oriented in an optimal way of J-aggregation.27,28
700 cm−1 from the absorption peak of the tetracene monomer appearing at 473 nm. The results suggest that J-aggregation occurs, where the molecules arrange into a slanted stack and the transition to the lower couple excited state of molecules is allowed. Accordingly, the absorption is red-shifted and intensely narrow. The molecules in the nanoparticles may be oriented in an optimal way of J-aggregation.27,28
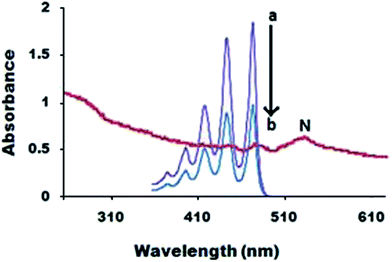 | ||
| Fig. 3 UV-vis absorption spectra of the TNPs in an aqueous dispersion (N) and a homogeneous solution of tetracene in THF (a and b). | ||
The fluorescence excitation and emission spectra of the dilute solution of isolated tetracene molecules in THF (λex/λem = 441/477 nm) and TNPs in an aqueous suspension (λex/λem = 318/533 nm) are shown in Fig. 4. The figure reveals that the excitation spectrum of the aqueous suspension of TNPs (spectrum C) is a blue shifted, broad band in comparison with the structured excitation spectrum of the tetracene monomer in THF solution (spectrum A). The fluorescence spectrum of the nanostructure (spectrum D) is a broad, structureless band with a maximum at 533 nm and is red shifted from that of the monomer emission of the tetracene in THF solution (spectrum B). The quantum efficiency of the aqueous suspension of the TNPs determined using a solution of quinine sulphate in H2SO4 as standard (ϕf = 0.725) is higher than the quantum efficiency of the solution of tetracene (ϕf = 0.17).29 Thus the TNPs exhibit Aggregation Induced Enhanced Emission (AIEE) like other polynuclear aromatic hydrocarbons.25 The Stokes shift, estimated as a difference between the excitation and fluorescence energy for the TNP suspension, is Δ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 12
= 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 684.810 cm−1. This value is significantly larger than the Stokes shift of the dilute solution of tetracene in THF which is Δ
684.810 cm−1. This value is significantly larger than the Stokes shift of the dilute solution of tetracene in THF which is Δ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 1711.376 cm−1. The observed large Stokes shift of the TNPs is attributed to the aggregation of the molecules by interaction between the slanted stacked neighbouring molecules, owing to the gradual increase in the excitonic coupling effect, by which the exciton relaxes to an energetically lower lying excited state. Hence, the emission of the TNPs originates from a lower lying excited state as compared to the isolated tetracene molecules.25 As a result, TNPs with a large Stokes shift lead to an enhanced emission of their aggregated molecules.
= 1711.376 cm−1. The observed large Stokes shift of the TNPs is attributed to the aggregation of the molecules by interaction between the slanted stacked neighbouring molecules, owing to the gradual increase in the excitonic coupling effect, by which the exciton relaxes to an energetically lower lying excited state. Hence, the emission of the TNPs originates from a lower lying excited state as compared to the isolated tetracene molecules.25 As a result, TNPs with a large Stokes shift lead to an enhanced emission of their aggregated molecules.
 | ||
| Fig. 4 Excitation (A) and fluorescence (FL) (B) spectra of tetracene in THF, excitation (C) and FL (D) spectra of the TNP suspension. | ||
3.3 Selective recognition of anionic fast green FCF by TNPs
The CTAB stabilized TNPs have a positively charged surface and hence can be used as a fluorescent sensor to recognize anions. To explore the selectivity of the proposed method the fluorescence response of the TNPs in an aqueous suspension was examined in the presence of several co-existing anions such as CH3COO−, NO3−, IO3−, Br−, SO42−, CO32−, BrO3−, Cl−, ClO3−, SO32−, F− and erythrosine dye solution (40 µg L−1) and in the presence of the fast green FCF dye (7 µg L−1). Fig. 5 is the spectral pattern which reveals that the fluorescence intensity of the TNPs did not respond to quenching appreciably by the addition of other co-existing anions, however the fluorescence of the TNPs was significantly quenched by fast green FCF which clearly indicates that only fast green FCF was involved in strong interactions with the TNPs, resulting in a method which is more sensitive and precise for the determination of the fast green FCF dye.3.4 Fluorescence lifetime of the TNPs
Fig. 6 presents the fluorescence decay profile of the dilute solution of tetracene in THF (I) and the aqueous suspension of the TNPs (II). The lifetime estimate from the decay profile of the nanoparticles of tetracene (2.92 ns) is relatively shorter than that of its solution with THF (4.44 ns). The lifetime estimate from the decay profile of TNPs recorded with increasing amounts of fast green FCF in aqueous solution is given in Table S1.† | ||
| Fig. 6 Fluorescence decay profiles of the dilute solution of tetracene in THF (I) and the aqueous suspension of the TNPs (II). | ||
3.5 Nature and mechanism of the fluorescence quenching process
The quenching of the fluorescence intensity of a fluorophore by a competing deactivating process resulting from the specific interaction between the fluorophore and the quencher substance is known as fluorescence quenching. Several mechanisms have been proposed for the fluorescence quenching of nanoparticles which include non-radiative recombination pathways, energy transfer, charge diversion, electron transfer processes, collisional quenching and ground state complex formation by surface adsorption. Quenching of a fluorophore arising from the formation of a non-fluorescent complex between a fluorophore and a foreign molecule is known as static quenching. In static quenching, two molecules interact by proton-coupled electron transfer to form hydrogen bonds. In aqueous solutions, electrostatic, steric and hydrophobic forces control the formation of the hydrogen bonds. When this complex absorbs energy from light, the excited state immediately returns to the ground state without emission of a photon and the molecules do not emit fluorescent light.30In the present paper, time resolved fluorescence spectroscopy was employed to decide the nature of the quenching of fast green FCF. The Stern–Volmer plot predicts, if quenching is dynamic in nature, then the plots of relative intensities and relative lifetimes should be identical, and if quenching is static in nature, the lifetime of the probe, in this case the TNPs, will remain unchanged.31
The photophysical quenching experiments using the following Stern–Volmer relations are applied to describe the quenching processes whereby the fluorescence intensity and lifetime data of the nanoparticles are fitted to eqn (1) and (2)
 | (1) |
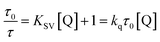 | (2) |
The fluorescence emission spectra of the TNPs were recorded with increasing amounts of the fast green FCF dye in an aqueous solution (1, 2, 3, 4, 5, 6, 7 µg L−1) and without the fast green FCF dye (see Fig. S1†). The fluorescence intensity of the probe, in this case the TNPs, was quenched significantly and regularly on the addition of the fast green FCF solution in the concentration range of 0.5–7.0 µg L−1. The quenching results fit into the conventional linear Stern–Volmer equation of fluorescence. Fig. 7 shows a plot of the changes in the fluorescence intensity  versus the various concentrations of the fast green FCF dye (blue line). Similarly the variation of lifetime
versus the various concentrations of the fast green FCF dye (blue line). Similarly the variation of lifetime 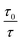 in the presence of the fast green FCF dye (red line) is also shown in the same figure. The figure predicts that the fluorescence intensity decreases while the excited state lifetime remains unchanged with increasing fast green FCF concentration, which suggests that a ground state complex was formed between the TNPs and the fast green FCF dye. The nature of the quenching process of the nanoparticles by fast green FCF described here is entirely static. The obtained experimental data for fast green FCF fitted well to the following empirical equation.
in the presence of the fast green FCF dye (red line) is also shown in the same figure. The figure predicts that the fluorescence intensity decreases while the excited state lifetime remains unchanged with increasing fast green FCF concentration, which suggests that a ground state complex was formed between the TNPs and the fast green FCF dye. The nature of the quenching process of the nanoparticles by fast green FCF described here is entirely static. The obtained experimental data for fast green FCF fitted well to the following empirical equation.
| F0/F = 0.25116x + 1 | (3) |
 | ||
Fig. 7 Comparative Stern–Volmer plot of  versus the addition of different amounts of the fast green FCF dye solution (0, 1, 2, 3, 4, 5, 6, 7 µg L−1). versus the addition of different amounts of the fast green FCF dye solution (0, 1, 2, 3, 4, 5, 6, 7 µg L−1). | ||
The linear relationship in the range of 0.5–7.0 µg L−1 has a correlation coefficient of R2 = 0.9990 (n = 3). The limit of detection is based on the definition by equation,20
| LOD = 3.3σ/k, | (4) |
As per the binding mode, the CTAB stabilizing the surface of the tetracene nanoparticles are bound to the fast green FCF by an electrostatic interaction, which forms a stable non-fluorescent micelle complex in the ground state, which results in the fluorescence quenching of the TNPs.
3.6 Fluorescent sensor for selective recognition of fast green FCF in environmental samples
A novel fluorescence quenching method was fruitfully applied to determine the presence of the fast green FCF dye by using commercially available samples such as fruit juices and soft drinks. These real sample analyses were carried out by using the same method which was used for the linearity experiments. The obtained results are summarized in Table 2 and it was found that the satisfactory recoveries were in the linear range. The precision was ascertained by calculating the relative standard deviation (RSD) of three replicate determinations. Recovery values obtained are in the range of 99.60–100.25%, which demonstrated that the method based on the fluorescence quenching of the TNPs can effectively recognize the fast green FCF dye over other co-existing ions in aqueous media.| Analyzed sample | Amount of standard fg FCF dye added (µg L−1) | Total fg FCF dye found (n = 3) (µg L−1) | Recovery of fg FCF dye added (average%) | RSD (%) | |
|---|---|---|---|---|---|
| a fg FCF – fast green FCF dye. | |||||
| Fruit juices | Sample no. 1 | 3.0 | 3.01 | 100.099 | 0.234 |
| 4.0 | 4.003 | ||||
| 5.0 | 4.98 | ||||
| Sample no. 2 | 2.0 | 2.016 | 100.210 | 0.253 | |
| 4.0 | 4.003 | ||||
| 6.0 | 5.98 | ||||
| Soft drinks | Sample no. 1 | 3.0 | 2.987 | 99.665 | 0.267 |
| 4.0 | 3.989 | ||||
| 5.0 | 4.985 | ||||
| Sample no. 2 | 3.0 | 3.983 | 99.72 | 0.366 | |
| 5.0 | 4.992 | ||||
| 6.0 | 5.983 | ||||
4. Conclusions
As prepared CTAB stabilized TNPs are recognizing the fast green FCF dye over other coexisting anions and the erythrosine dye in aqueous media. We have developed a novel, simple and convenient method for the determination of the fast green FCF dye based on the fluorescence quenching of the CTAB stabilized TNPs. The possible quenching mechanism is owing to the electrostatic interaction which forms a ground state complex between the nanoparticles and fast green FCF. The calibration curve is linear over the concentration range 0.5–7.0 µg L−1 with a correlation coefficient of 0.999 and an extremely low detection limit of 0.136 µg L−1. Further studies may open new applications of organic nano-materials based on a fluorescence quenching approach in the direct or simultaneous detection of other food dyes.Acknowledgements
We gratefully acknowledge the Department of Science and technology, Delhi and the University Grants Commission (UGC), Delhi for providing funds to the Chemistry department under FIST and SAP-DRS Phase-II program, respectively.References
- C. O’Neill, F. R. Hawkes, D. L. Hawkes, N. D. Lourenco, H. M. Pinheiro and W. Delee, J. Chem. Technol. Biotechnol., 1999, 74, 1009–1018 CrossRef.
- H. Moawad, W. M. El-Rahim and M. Khalafallah, J. Basic Microbiol., 2003, 43, 218–229 CrossRef CAS PubMed.
- Y. Wu, M. Zhang, H. Zhao, S. Yang and A. Arkin, RSC Adv., 2014, 4, 61256–61267 RSC.
- A. Shokrollahi and T. Roozestan, Anal. Methods, 2013, 5, 4824–4831 RSC.
- A. Mittal, D. Kaur and J. Mittal, J. Hazard. Mater., 2009, 163, 568–577 CrossRef CAS PubMed.
- K. Yuet-Wan Lok, W.-Y. Chung, I. F. F. Benzie and J. Woo, Food Addit. Contam., Part B, 2010, 3(3), 148–155 CrossRef CAS PubMed.
- N. Yoshioka and K. Ichihashi, Talanta, 2008, 74, 1408–1413 CrossRef CAS PubMed.
- O. Sha, X. Zhu, Y. Feng and W. Ma, Food Chem., 2015, 174, 380–386 CrossRef CAS PubMed.
- M. J. Culzonia, A. V. Schenonea, N. E. Llamas, M. Garrido, M. S. Di Nezio, B. S. Fernández Band and H. C. Goicoechea, J. Chromatogr. A, 2009, 1216, 7063–7070 CrossRef PubMed.
- E. C. Vidotti, W. F. Costa and C. C. Oliveira, Talanta, 2006, 68, 516–521 CrossRef CAS PubMed.
- M. Ma, X. Luo, B. Chen, S. Su and S. Yao, J. Chromatogr. A, 2006, 1103, 170–176 CrossRef CAS PubMed.
- K. Fraige, N. A. Assuncao, R. D. S. Pinto and E. Carrilho, J. Liq. Chromatogr. Relat. Technol., 2009, 32, 1862–1878 CrossRef CAS PubMed.
- S. P. Alves, D. M. Brum, E. C. B. de Andrade and A. D. P. Netto, Food Chem., 2008, 107, 489–496 CrossRef CAS PubMed.
- R. Colombo, F. M. Lancas and J. H. Yariwake, J. Chromatogr. A, 2006, 1103, 118–124 CrossRef CAS PubMed.
- F. Gosetti, V. Gianotti, S. Angioi, S. Polati, E. Marengo and M. C. Gennaro, J. Chromatogr. A, 2004, 1054, 379–387 CAS.
- S. Tsuji, K. Yoshii and Y. Tonogai, J. Chromatogr. A, 2006, 1101, 214–221 CrossRef CAS PubMed.
- F. I. de Andrade, M. I. F. Guedes, I. G. P. Vieira, F. N. P. Mendes, P. A. S. Rodrigues, C. S. C. Maia, M. M. M. Avila and L. D. M. Ribeiro, Food Chem., 2014, 157, 193–198 CrossRef PubMed.
- M.-H. Sorouraddin and A. R. M. Saadati, Food Chem., 2011, 127, 308–313 CrossRef CAS PubMed.
- D. K. Dalavi, D. P. Bhopate, A. S. Bagawan, A. H. Gore, N. K. Desai, A. A. Kamble, P. G. Mahajan, G. B. Kolekar and S. R. Patil, Anal. Methods, 2014, 6, 6948–6955 RSC.
- L. Wang, L. Wang, T. Xia, L. Dong, G. Bian and H. Chen, Anal. Sci., 2004, 20, 1013–1017 CrossRef CAS.
- R. Dua, S. Shrivastava, S. L. Shrivastava and S. K. Srivastava, Middle East J. Sci. Res., 2012, 11(7), 846–855 CAS.
- Y. Lei, Q. Liao, H. Fu and J. Yao, J.Phys. Chem. C, 2009, 113, 10038–10043 CAS.
- A. G. L. Olive, A. D. Guerzo, C. Schafer, C. Belin, G. Raffy and C. Giansante, J. Phys. Chem. C, 2010, 114, 10410–10416 CAS.
- P. G. Mahajan, N. K. Desai, D. K. Dalavi, D. P. Bhopate, G. B. Kolekar and S. R. Patil, J. Fluoresc., 2015, 25, 31–38 CrossRef CAS PubMed.
- D. P. Bhopate, P. G. Mahajan, K. M. Garadkar, G. B. Kolekar and S. R. Patil, RSC Adv., 2014, 4, 63866–63874 RSC.
- Y. Dieckmann, H. Colfen, H. Hofmann and A. Petri-Fink, Anal. Chem., 2009, 81, 3889–3895 CrossRef CAS PubMed.
- B.-K. An, S.-K. Kwon, S.-D. Jung and S. Y. Park, J. Am. Chem. Soc., 2002, 124, 14410–14415 CrossRef CAS PubMed.
- S. Li, H. L. He, F. Xiong, Y. Li and G. Yang, J. Phys. Chem. B, 2004, 108, 10887–10892 CrossRef CAS.
- A. M. Muller, Y. S. Avlasevich, K. Mullen and C. J. Bardeen, Chem. Phys. Lett., 2006, 421, 518–522 CrossRef PubMed.
- B. Zhang, W. Diao, C. Bi, J. Sun, G. Han, Y. Shi, L. Sheng, G. Yin and L. Pu, J. Fluoresc., 2012, 22, 1–7 CrossRef CAS PubMed.
- M. K. Seery, N. Fay, T. McCormac, E. Dempsey, R. J. Forster and T. E. Keyes, Phys. Chem. Chem. Phys., 2005, 7, 3426–3433 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09835a |
| This journal is © The Royal Society of Chemistry 2015 |



