Simultaneous nitrification and denitrification using a polypyrrole/microbial cellulose electrode in a membraneless bio-electrochemical system
Abstract
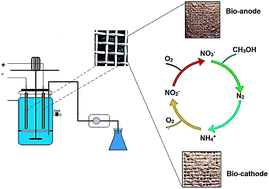
In this study, the feasibility of ammonium and total nitrogen (TN) removal from aqueous solution using a simultaneous nitrification and denitrification process was studied in a membraneless (single chamber) bio-electrochemical system with a novel electrode. The main objectives were to synthesize a polypyrrole/microbial cellulose (PPy/MC) composite and utilize it as a novel electrode material. To determine the mechanical properties of PPy/MC, the tensile strength and Young’s modulus were investigated. A biofilm was prepared using the fabricated electrode during the first few weeks. Effective parameters such as initial ammonium concentrations (NH4+ ∼ 15–150 mg N L−1), HRT (6–72 h), carbon/nitrogen ratio (C/N ratio ∼ 0–4), current intensity (2–10 mA), and pH (6.5–8.5) were evaluated. The following optimum values were obtained: HRT, 24 h; C/N ratio, 2; electric current, 6 mA; and pH, 7–7.5 at a constant ammonium concentration of 77.77 mg N L−1. It can be concluded from the experimental data that under optimal conditions about 97.42 and 62.47% of ammonium and TN were removed successfully, respectively.

 Please wait while we load your content...
Please wait while we load your content...