Synthesis of graphene/α-Fe2O3 composites with excellent electromagnetic wave absorption properties†
Abstract
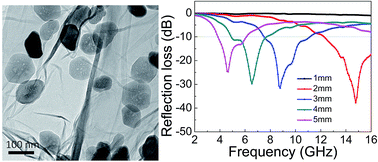
A novel three-dimensional (3D) composite based on reduced graphene oxide (rGO)/Fe2O3 was prepared by a one-pot hydrothermal method. Fe2O3 nanoparticles were either attached on the surface of graphene sheets or coated uniformly in the graphene sheets. The resulting composite is found to self-assemble to form a 3D network via hydrothermal treatment. Our results indicate that the as-prepared Fe2O3 nanoparticles show a porous morphology, which results in the rGO/Fe2O3 composites exhibiting excellent microwave absorbing properties in the range of 2–16 GHz and are therefore expected to be a promising candidate as microwave absorbing materials.

 Please wait while we load your content...
Please wait while we load your content...