Environmentally benign synthesis of high-quality, band gap-tunable, homogeneous Te/Se alloyed nanowires†
Chongjian Zhoua,
Ke Wanga,
Chaochao Dunc,
Qiong Wanga,
Zhongqi Shi*a,
Guiwu Liu*b and
Guanjun Qiaoab
aState Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an 710049, China. E-mail: zhongqishi@mail.xjtu.edu.cn
bSchool of Materials Science and Engineering, Jiangsu University, Zhenjiang 212013, China. E-mail: gwliu76@ujs.edu.cn
cCenter for Nanotechnology and Molecular Materials, Department of Physics, Wake Forest University, Winston-Salem, NC 27109, USA
First published on 31st July 2015
Abstract
Ultrathin trigonal Te/Se alloyed nanowires with a tunable composition and band gap were fabricated using a nonhazardous reducing agent, ascorbic acid. The as-synthesized nanowires displayed a tunable direct band gap (3.39 to 3.78 eV) and indirect band gap (1.99 eV to 2.93 eV).
As typical p-type semiconductors with narrow band gaps and high activity in hydration and oxidation reactions, Te, Se and Te/Se alloys are excellent candidates in the fields of gas sensing,1 piezoelectrics,2 thermoelectrics3–7 and photoconductivity.8,9 Te/Se alloys are composed of Te–Se atom-bonded helical chains with Te and Se atoms randomly united via van der Waals forces.10,11 Both the trigonal Te (t-Te) and Se (t-Se) crystallize in space group D43 which has three atoms per unit cell arranged helically along the c axis,12 leading to almost identical properties. However, slight differences such as amorphous-to-crystalline transition temperature exist, which actually result in difficulty in controlling the morphology of t-Te/Se alloyed nanocrystals synthesized using a solution phase method11 for their wide applications.
One commonly utilized solution-based synthesis method to fabricate metal t-Te/Se alloyed nanocrystals employed hydrazine as the reducing agent,10,11,13–17 which is unfortunately explosive, pyrophoric, and carcinogenic. Hydroxylamine and sodium borohydride as alternative reducing agents are tolerable but still moderately hazardous.18–20 Moreover, most of the reactions in previous studies involved using the expensive orthotelluric acid as the Te precursor.10,13,18,21 In contrast, ascorbic acid (vitamin C) is believed to be a relatively safe reducing agent that has been preliminarily used in many solution based synthesis processes.22–24 So far, few reports have focused on the synthesis of Te/Se alloyed nanowires using ascorbic acid as a reducing agent. Moreover, as they are promising thermoelectric and photoconductive materials, the band gap engineering of Te/Se nanowires is essential. However, precise control of the band gap in Te/Se nanowires is difficult since the band gap normally changes irregularly with increased Se percentage due to the poor morphology control of the alloyed nanowire.11
Herein, we put forward an environmentally benign and scalable one-pot strategy to fabricate high-quality t-Te/Se nanowires with well controlled aspect ratios using ascorbic acid as a reducing agent. The synthesis method used Te nanowires with different aspect ratios as sacrificial templates. By injecting the Se precursor at a certain temperature, high quality ultrathin Te/Se alloyed nanowires with diameters of less than 10 nm were synthesized. Unlike in previous reports,11 the as-synthesized alloyed nanowires were determined to have a single phase, instead of a simple core–shell structure, which exhibited a continuously tunable band gap and could be used as photoconductive and thermoelectric materials.
Taking the typical Te0.5Se0.5 nanowires with an average diameter of around 10 nm as an example, the synthesis of the t-Te/Se nanowires was accomplished as follows: (1) synthesis of Te nanowires with an average diameter of around 6 nm: 3 mmol tellurium dioxide (TeO2), 0.75 g polyvinylpyrrolidone (PVP, Mw ∼ 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), and 10 mmol KOH were dissolved in 30 mmol ethylene glycol (EG) with vigorous stirring, which resulted in a cloudy white solution. A transparent yellow solution was obtained after heating to 120 °C. Then 6 ml of 1.89 M aqueous ascorbic acid solution was rapidly injected and the solution turned an opaque black within 1 min. The reaction proceeded for 3 h under a protective N2 atmosphere to allow the Te precursor to convert to ultrathin Te nanowires. (2) Formation of Te@Se core–shell nanowires: a Se precursor solution was prepared by dissolving 3 mmol selenic acid (H2SeO3) and 0.1 g PVP in 10 ml EG, followed by heating to ∼80 °C in a separate vial. Meanwhile, the Te solution was cooled down to 90 °C. The Se solution was injected into the Te reaction solution, and then 2.5 ml 18 M hydrochloric acid (HCl) dissolved in 2.5 ml EG was also injected into the solution to form the Te@Se core–shell nanowires. (3) Transformation of the Te@Se core–shell nanowires into Te0.5Se0.5 alloyed nanowires: the solution temperature was raised to 110 °C for another hour, after which the reaction solution was cooled down to room temperature naturally. Finally, the Te0.5Se0.5 alloyed nanowires were successfully obtained. Te/Se alloyed nanowires with a tunable composition can also be synthesized and their yields were higher than 90%. Similarly, by appropriate control of the synthesis procedure, Te/Se nanowires with a tunable aspect ratio can be fabricated by simply tuning the aspect ratio of the Te nanowire template (Fig. S1†). Details of the experimental process and the characterizations can be found in the ESI.†
000), and 10 mmol KOH were dissolved in 30 mmol ethylene glycol (EG) with vigorous stirring, which resulted in a cloudy white solution. A transparent yellow solution was obtained after heating to 120 °C. Then 6 ml of 1.89 M aqueous ascorbic acid solution was rapidly injected and the solution turned an opaque black within 1 min. The reaction proceeded for 3 h under a protective N2 atmosphere to allow the Te precursor to convert to ultrathin Te nanowires. (2) Formation of Te@Se core–shell nanowires: a Se precursor solution was prepared by dissolving 3 mmol selenic acid (H2SeO3) and 0.1 g PVP in 10 ml EG, followed by heating to ∼80 °C in a separate vial. Meanwhile, the Te solution was cooled down to 90 °C. The Se solution was injected into the Te reaction solution, and then 2.5 ml 18 M hydrochloric acid (HCl) dissolved in 2.5 ml EG was also injected into the solution to form the Te@Se core–shell nanowires. (3) Transformation of the Te@Se core–shell nanowires into Te0.5Se0.5 alloyed nanowires: the solution temperature was raised to 110 °C for another hour, after which the reaction solution was cooled down to room temperature naturally. Finally, the Te0.5Se0.5 alloyed nanowires were successfully obtained. Te/Se alloyed nanowires with a tunable composition can also be synthesized and their yields were higher than 90%. Similarly, by appropriate control of the synthesis procedure, Te/Se nanowires with a tunable aspect ratio can be fabricated by simply tuning the aspect ratio of the Te nanowire template (Fig. S1†). Details of the experimental process and the characterizations can be found in the ESI.†
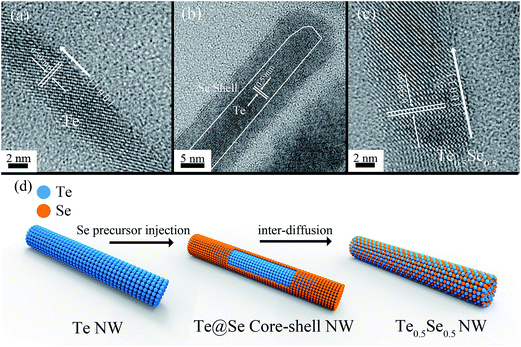
Fig. 1(a) and (d) show the transmission electron microscopy (TEM) images of the Te and Te0.5Se0.5 nanowires, respectively. It can be seen that both are distributed evenly, with homogeneous diameters. The HRTEM image (Fig. 1(b)) of an individual Te nanowire reveals the highly crystalline nature and the [001] preferential lattice plane of Te, which is in agreement with the previous report.25 As shown in Fig. 1(c), the mean diameter of the Te nanowires is in a range of 6–7 nm, with the lateral length reaching around 975 ± 47 nm. On the other hand, the TEM and HRTEM images of the Te0.5Se0.5 nanowires (Fig. 1(d) and (e)) demonstrate that the nanowires retained the morphology and the preferential growth direction [001] of the pristine Te nanowires. The single crystallization of the Te0.5Se0.5 nanowires is also verified, as shown in Fig. 1(e). Compared with the Te nanowires, the mean diameter of the Te0.5Se0.5 nanowires is slightly increased from 6–7 nm to 9–10 nm and the length reaches values as high as 1502 ± 82 nm. In fact, the nanowires are quite flexible, and the TEM images might lead to some unreliable values because the nanowires deposited on the TEM grid are not completely attached to the surface of the grid, resulting in a deviation from the actual length values. However, from a statistical perspective, the deviation of the length measurements of the nanowires on the TEM grid can be neglected when a large number of nanowires are measured. To the best of our knowledge, they are the longest and thinnest Te/Se alloyed nanowires reported so far.10,11,13,18,21,26 The diameter and length expansions mainly come from the epitaxial growth of a Se shell on the outside of the fabricated Te nanowires after injecting the Se precursor (Fig. 2(b)). Obviously, the Se shell growth around the Te nanowire is amorphous and becomes crystalline with rising temperature. As Se and Te belong to the same group, Se atoms are allowed to diffuse into the fabricated Te nanowires along the Te/Se interface, leading to Te/Se alloyed nanowires with preserved morphology and a single crystalline nature. The whole growth mechanism is described in Fig. 2(d). To further confirm the fabrication process of the Te/Se alloyed nanowires, energy dispersive spectroscopy (EDS) elemental mapping (Fig. 3) and high-angle annular dark-field (HAADF) imaging (Fig. S2†) of the selected Te0.5Se0.5 nanowires were performed. Theoretically, brightness differences would be observed under HAADF mode if Te and Se are inhomogeneously distributed. However, as can be seen in Fig. S2,† no brightness difference is observed, suggesting that the Te and Se elements are indeed distributed homogeneously. The stoichiometric composition ratio of the Te0.5Se0.5 nanowires was elucidated to be around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 (Fig. S3†), which matches well with the initial precursor ratio as expected.
0.9 (Fig. S3†), which matches well with the initial precursor ratio as expected.
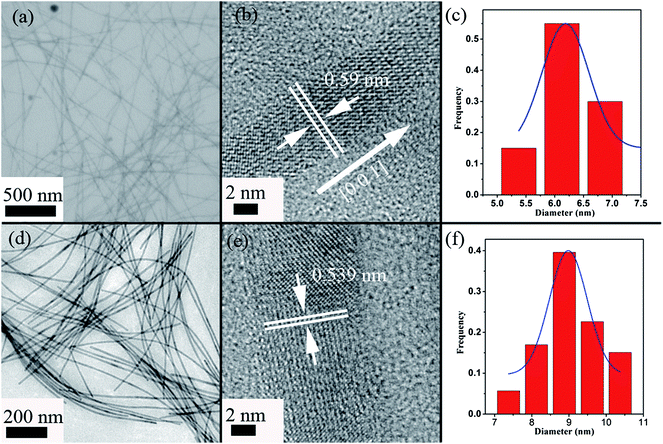 | ||
| Fig. 1 TEM, HRTEM images and diameter distributions of the Te nanowires (a–c) and Te0.5Se0.5 nanowires (d–f). | ||
 | ||
Fig. 3 EDS elemental mapping of the synthesized Te0.5Se0.5 nanowires, which reveals a homogeneous distribution of the Te and Se atoms with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9. 0.9. | ||
In order to systematically verify the single phase of the Te/Se alloys instead of a simple core–shell structure, the X-ray diffraction (XRD) patterns and TEM images of the synthesized Te nanowires and Te/Se nanowires were studied, and are shown in Fig. 4 and S4, respectively.† The XRD pattern of the synthesized Te nanowires adopted as the sacrificial template matches well with that of the t-Te (JCPDS 36-1452). On the contrary, the peaks in the XRD pattern of the Te/Se alloyed nanowires are shifted continuously to a higher angle (compared with the Te nanowires) with increasing Se content. It is apparent that the XRD pattern of the Te–Se alloyed nanowires shows a single phase instead of a superimposition of the pure Te and Se phases. In a previous report, the synthesis of Te–Se nanowires using Te nanowires as a template in water did not produce a single phase nanowire because the water was limited to a maximum reflux temperature of 100 °C at ambient pressure,21 therefore a mixed phase of SexTey–Te nanowires was fabricated. Here, by using EG as the solvent, the synthesis temperature can be raised high enough (>110 °C) to empower the Se atoms to diffuse uniformly into the Te nanowires. In fact, when the reaction temperature was below 110 °C, mixed phase nanowires also existed in our product (Fig. S5†). With the increase in temperature, it is believed that the Se atoms were able to diffuse into the Te spiral chains until they were uniformly distributed in the Te/Se nanowires, as shown in Fig. 2. Furthermore, from the XRD results shown in Fig. 4, it was found that the diffraction peaks of Te1−xSex nanowires, especially Te0.5Se0.5, are relatively broader than those of the Te nanowires, and in fact the Te nanowires are thinner than their counterpart Te1−xSex. This phenomenon might also be attributed to the breaking of long-range atom order by the random distribution of Se atoms in the Te nanowires. The wave-like striped pattern in the TEM dark field image of a single Te0.5Se0.5 nanowire (Fig. S6†) also indicates the stress induced by the inter-diffusion of Se into Te, which is believed to result from not only the high surface-to-volume ratio, but also the highly disordered distribution of Se atoms along the nanowire. In fact, due to the Te0.5Se0.5 nanowires possessing a single phase crystallization instead of a core–shell structure, no orientation contrast should theoretically be observed under a dark field mode. However, the random distribution of Se and Te atoms might introduce a lattice mismatch around the neighboring lattice, leading to an uneven localized force and thus the wave-like striped patterns.
As discussed above, the present Te/Se alloyed nanowire is a kind of pseudo-binary crystal, which means it is not only a simple mixture of Te and Se atoms, but also exists as a pseudo-copolymer, with pseudo-covalent Te–Se bonds. Raman spectroscopy was employed to quantitatively validate the intrinsic vibration properties of the pseudo-binary Te/Se alloyed nanowires. As shown in Fig. 5, four different compositions were investigated, i.e. Te nanowires, and Te/Se alloyed nanowires with atom ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The Te nanowires present two peaks located at 122 cm−1 and 146 cm−1, which correspond to the A1 and ETO vibrational modes, respectively.27 Obviously, the Te nanowires display a single Te–Te vibrational mode behavior. However, the Te/Se alloyed nanowires display a multi-mode vibrational behavior. For example, when the Te
2. The Te nanowires present two peaks located at 122 cm−1 and 146 cm−1, which correspond to the A1 and ETO vibrational modes, respectively.27 Obviously, the Te nanowires display a single Te–Te vibrational mode behavior. However, the Te/Se alloyed nanowires display a multi-mode vibrational behavior. For example, when the Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se ratio is 2
Se ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the peak around 122 cm−1 seems to disappear, while new peaks at 143 cm−1 and 174 cm−1 are observed. The peak at 143 cm−1 which corresponds to the A2 symmetry mode is attributed to the Te–Se vibrational mode which can only be found in Te–Se alloy compounds.28 The peak at 174 cm−1 is ascribed to the Se–Se vibrational mode. In fact, the Te–Te mode (122 cm−1) also exists, however it is less infrared-inactive, such that its intensity becomes too small to detect.28 The Te–Se and Se–Se modes are continuously blue shifted with increasing Se percentage. However, when the ratio of Te
1, the peak around 122 cm−1 seems to disappear, while new peaks at 143 cm−1 and 174 cm−1 are observed. The peak at 143 cm−1 which corresponds to the A2 symmetry mode is attributed to the Te–Se vibrational mode which can only be found in Te–Se alloy compounds.28 The peak at 174 cm−1 is ascribed to the Se–Se vibrational mode. In fact, the Te–Te mode (122 cm−1) also exists, however it is less infrared-inactive, such that its intensity becomes too small to detect.28 The Te–Se and Se–Se modes are continuously blue shifted with increasing Se percentage. However, when the ratio of Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se reached 1
Se reached 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the A1 mode of pure Te reemerged. According to the previous report,28 the reappearance of the A1 mode can be explained by the decreased intensity of the Te–Se vibrational mode. When the Se atom percentage in the Te/Se alloy exceeds 50%, the deviated second optical band frequency that is far away from the pure Te vibration also results in the reappearance of the A1 mode. Importantly, it is worth pointing out that this is the first time a transition from single mode vibrational behavior to multi-mode vibrational behavior has been observed, with a continuous Raman blue shift as the Se percentage increased in the Te/Se alloyed nanowires.
2, the A1 mode of pure Te reemerged. According to the previous report,28 the reappearance of the A1 mode can be explained by the decreased intensity of the Te–Se vibrational mode. When the Se atom percentage in the Te/Se alloy exceeds 50%, the deviated second optical band frequency that is far away from the pure Te vibration also results in the reappearance of the A1 mode. Importantly, it is worth pointing out that this is the first time a transition from single mode vibrational behavior to multi-mode vibrational behavior has been observed, with a continuous Raman blue shift as the Se percentage increased in the Te/Se alloyed nanowires.
UV-vis absorption spectra were obtained to investigate the band gap variation of Te/Se alloyed nanowires with different compositions (Fig. 6). The spectrum of the Te nanowires is almost the same as in the previous literature.29 For the Te nanowires, the absorption peaks located at 365 nm (3.39 eV) and 621 nm (1.99 eV) correspond to the direct band gap and indirect band gap, respectively. With the formation of Te/Se alloyed nanowires and the increased Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se molar ratio, a blue shift of the UV-vis absorption spectra is clearly observed. For example, for a Te
Se molar ratio, a blue shift of the UV-vis absorption spectra is clearly observed. For example, for a Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se molar ratio of 1
Se molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the direct and indirect band gaps become 3.78 eV and 2.93 eV, respectively. In a previous report,11 an irregular change of the band gap in the Te/Se nanowires with increased Se atom percentage was found, and the authors attributed that to the poor morphology control of the nanostructures. Here, based on the well-controlled synthesis method in our study, the synthesis of tunable compositions and continued adjustment of the band gap for the ultrathin Te/Se alloy nanowires were achieved, which is critical for their use as photoconductive and thermoelectric materials.
2, the direct and indirect band gaps become 3.78 eV and 2.93 eV, respectively. In a previous report,11 an irregular change of the band gap in the Te/Se nanowires with increased Se atom percentage was found, and the authors attributed that to the poor morphology control of the nanostructures. Here, based on the well-controlled synthesis method in our study, the synthesis of tunable compositions and continued adjustment of the band gap for the ultrathin Te/Se alloy nanowires were achieved, which is critical for their use as photoconductive and thermoelectric materials.
In summary, a scalable and environmentally benign solution-based method to synthesize homogeneous and composition-tunable Te/Se alloyed nanowires was proposed, which was achieved by using ascorbic acid as the reducing agent instead of the highly toxic and explosive hydrazine. The composition of the Te/Se alloyed nanowires can be easily controlled by adjusting the molar ratio of TeO2 to H2SeO3 during the reaction. The length of the ultrathin Te/Se alloyed nanowires with tunable compositions can reach values as high as 1502 nm while the diameter remains less than 10 nm. The well controlled Te/Se alloyed nanowires also displayed a tunable direct band gap changing from 3.39 to 3.78 eV and an indirect band gap changing from 1.99 eV to 2.93 eV.
These band gap tunable Te/Se nanowires are anticipated to be used as photoconductive materials. Moreover, due to the nearly identical reactivity of Te and Se elements, the Te/Se alloyed nanowires can also be used as a sacrificial template to synthesise other kinds of ternary nanowires, such as Cu–Te–Se, Ag–Te–Se, Pb–Te–Se and so on, all of which are promising thermoelectric materials.
Acknowledgements
This work was supported by the Program for New Century Excellent Talents in University (NCET-12-0454), the Program for Young Excellent Talents in Shaanxi Province (2013KJXX-50) and the Fundamental Research Funds for the Central University (XJJ2015104).References
- Z. Wang, L. Wang, J. Huang, H. Wang, L. Pan and X. Wei, J. Mater. Chem., 2010, 20, 2457–2463 RSC.
- T. Lee II, S. Lee, E. Lee, S. Sohn, Y. Lee, S. Lee, G. Moon, D. Kim, Y. S. Kim, J. M. Myoung and Z. L. Wang, Adv. Mater., 2013, 25, 2920–2925 CrossRef PubMed.
- G. Zhang, B. Kirk, L. A. Jauregui, H. Yang, X. Xu, Y. P. Chen and Y. Wu, Nano Lett., 2012, 12, 56–60 CrossRef CAS PubMed.
- K. Wang, H.-W. Liang, W.-T. Yao and S.-H. Yu, J. Mater. Chem., 2011, 21, 15057–15062 RSC.
- C. Dun, C. Hewitt, H. Huang, D. Montgomery, J. Xu and D. Carroll, Phys. Chem. Chem. Phys., 2015, 17, 8591–8595 RSC.
- C. Dun, C. Hewitt, H. Huang, J. Xu, D. Montgomery, W. Nie, Q. Jiang and D. L. Carroll, ACS Appl. Mater. Interfaces, 2015, 7, 7054–7059 CAS.
- F. Drymiotis, T. W. Day, D. R. Brown, N. A. Heinz and G. Jeffrey Snyder, Appl. Phys. Lett., 2013, 103, 143906 CrossRef PubMed.
- B. Gates, B. Mayers, B. Cattle and Y. Xia, Adv. Funct. Mater., 2002, 12, 219–227 CrossRef CAS.
- Z.-M. Liao, C. Hou, Q. Zhao, L.-P. Liu and D.-P. Yu, Appl. Phys. Lett., 2009, 95, 093104 CrossRef PubMed.
- B. B. Mayers, B. Gates, Y. Yin and Y. Xia, Adv. Mater., 2001, 13, 1380–1384 CrossRef CAS.
- S. Fu, K. Cai, L. Wu and H. Han, CrystEngComm, 2015, 17, 3243–3250 RSC.
- P. S. P. Boolchand, Phys. Rev. B: Solid State, 1973, 7, 57–60 CrossRef CAS.
- B. Zhou and J.-J. Zhu, Nanotechnology, 2006, 17, 1763–1769 CrossRef CAS.
- D. Qin, J. Zhou, C. Luo, Y. Liu, L. Han and Y. Cao, Nanotechnology, 2006, 17, 674–679 CrossRef CAS.
- H. Yang, J.-H. Bahk, T. Day, A. M. S. Mohammed, B. Min, G. J. Snyder, A. Shakouri and Y. Wu, Nano Lett., 2014, 14, 5398–5404 CrossRef CAS PubMed.
- H. Fang, H. Yang and Y. Wu, Chem. Mater., 2014, 26, 3322–3327 CrossRef CAS.
- Z. Lin, Z. Yang and H. Chang, Cryst. Growth Des., 2008, 8, 351–357 CAS.
- G. D. Moon, Y. Min, S. Ko, S. Kim, D. Ko and U. Jeong, ACS Nano, 2010, 4, 7283–7292 CrossRef CAS PubMed.
- T. J. Zhu, X. Chen, X. Y. Meng, X. B. Zhao and J. He, Cryst. Growth Des., 2010, 10, 3727–3731 CAS.
- H. Duan, D. Wang and Y. Li, Chem. Soc. Rev., 2015, 44, 5778–5792 RSC.
- H. Tao, X. Shan, D. Yu, H. Liu, D. Qin and Y. Cao, Nanoscale Res. Lett., 2009, 4, 963–970 CrossRef CAS PubMed.
- N. R. Jana, L. Gearheart and C. J. Murphy, J. Phys. Chem. B, 2001, 105, 4065–4067 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Adv. Mater., 2001, 13, 1389–1393 CrossRef CAS.
- H. Yang, S. W. Finefrock, J. D. A. Caballero and Y. Wu, J. Am. Chem. Soc., 2014, 136, 10242–10245 CrossRef CAS PubMed.
- G. Zhang, H. Fang, H. Yang, L. A. Jauregui, Y. P. Chen and Y. Wu, Nano Lett., 2012, 12, 3627–3633 CrossRef CAS PubMed.
- K. Sridharan, T. J. P. Muhamed Shafi Ollakkan and R. Philip, Carbon, 2013, 63, 263–273 CrossRef CAS PubMed.
- G. D. A. S. Pine, Phys. Rev. B: Solid State, 1970, 4, 356–371 CrossRef.
- R. Geick, E. F. Steigmeier and H. Auderset, Phys. Status Solidi, 1972, 54, 623–630 CrossRef CAS PubMed.
- J.-M. Song, J.-H. Zhu and S.-H. Yu, J. Phys. Chem. B, 2006, 110, 23790–23795 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09615d |
| This journal is © The Royal Society of Chemistry 2015 |