Cooling-induced formation of honeycomb patterns on pre-cast PMMA films at low temperatures†
Abstract
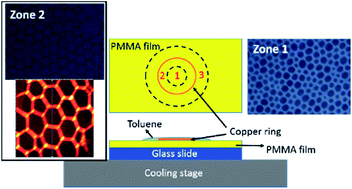
Using a copper ring to control the evaporation of toluene droplets on the surface of PMMA films at low temperatures, honeycomb patterns of various topologies are formed in different regions. The honeycomb with the largest pore size is around the center area, and the pore size decreases gradually as the distance to the center increases. The effects of the film thickness and the substrate temperature on the honeycomb structures are examined. The pore size increases with the decrease of the film thickness. Hexagonal networks are formed at the substrate temperature of ∼8 °C. The “breath figure” mechanism is used to explain the formation of the honeycomb structures. The patterns formed on PS films under the same experimental conditions are examined, which are significantly different from the patterns formed on PMMA films.

 Please wait while we load your content...
Please wait while we load your content...