UV irradiation-induced cross-linked bicarbonate anion exchange membranes based on vinylimidazolium-functionalized poly(arylene ether ketone)
Fanzhe Bua,
Chengji Zhao*a,
Baolong Wanga,
Na Zhanga,
Hao Lub,
Zhenzhen Caic,
Yurong Zhanga and
Hui Na*a
aAlan G. MacDiarmid Institute, College of Chemistry, Jilin University, Changchun 130012, PR China. E-mail: zhaochengji@jlu.edu.cn; huina@jlu.edu.cn; Fax: +86-431-85168870; Tel: +86-431-85168870
bDepartment of Oral Pathology, College of Hospital of Stomatology, Jilin University, Changchun 130012, PR China
cState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, PR China
First published on 24th June 2015
Abstract
A novel cross-linking strategy for imidazolium-functionalized poly(arylene ether ketone) containing tetramethyl groups, used as anion exchange membranes, is presented in this paper. The preparation of anion exchange membranes comprised of converting benzylic methyl to bromomethyl groups by a radical reaction, quaternization of the bromometylated poly(arylene ether ketone) with 1-vinylimidazole and subsequent anion exchange reaction. The photo-crosslinking reaction, induced by UV-light under the activation of a photo-initiator, was simple and controllable compared to the conventional cross-linking process via a multifunctional cross-linking agent. All the vinylimidazolium-founctionalized poly(arylene ether ketone) membranes showed good mechanical and thermal stabilities. Moreover, the cross-linked membranes exhibited better dimensional stabilities. The ionic conductivity of cross-linked membranes was still acceptable although that property decreased with UV irradiation time due to the compact cross-linked structure. For example, the VImPAEK-15 min-HCO3 membrane with irradiation time of 15 min showed a moderate bicarbonate anion conductivity of 0.021 S cm−1 and a low swelling ratio of 10.74% at 70 °C. The alkaline stability test under both high and relatively low alkaline conditions was carried out. Although the vinylimidazolium cation is unstable in strong alkaline solution, the cross-linked vinylimidazolium-based membranes are chemically stable in the bicarbonate anion form under relatively low alkaline conditions. The results showed that the cross-linked membrane induced by UV irradiation could be used for electrochemical devices that were operated at less extreme pHs.
Introduction
The enthusiasm for research on anion exchange membranes (AEMs) has rapidly grown in recently years, because it has attracted interest in a variety of applications, including solid-state alkaline fuel cells (AFCs),1,2 redox flow batteries,3 electrodialysis stacks,4 and metal–air batteries.5 These electrochemical or energy storage devices permit the use of inexpensive non-platinum catalysts (e.g., nickel, silver and carbon) for the oxygen reduction reaction to greatly reduce the cost of devices. AEM is one of the key components in AFCs, which could conduct the hydroxide, bicarbonate or carbonate anions through the polymer electrolyte. At present, quaternary ammonium (QA) based AEMs have been one of the most widely investigated.6,7 They are typically prepared by immersing the pre-formed membranes of chloro-/bromomethylated polymers into aqueous trimethylamine solution to complete the quaternization reaction. Various polymers with tethered QA groups have been investigated, including polysulfones,8 poly(arylene ether ketone)s,9 poly(phenylene oxide)s,10,11 polyphenylenes12 and poly(olefin)s.13 However, QA tethered polymer electrolytes traditionally exhibited low stability when operating under the harsh conditions (i.e. high pH above 60 °C). The degradation of QA cations was caused by the following pathways: (i) β-hydrogen Hofmann elimination (E2), (ii) ylide formation, or (iii) nucleophilic substitution at the α-carbon (SN2).14,15 Each pathway leads to a rapid decrease in ionic exchange capacity (IEC) and ionic conductivity.To circumvent the obstacle in the lack of stability and to achieve higher ionic conductivity of QA AEMs, considerable efforts have been made to develop non-QA AEMs containing imidazolium,16 phosphonium,17 guanidinium18,19 or quaternized DABCO cations.20 In particular, imidazolium functionalized AEMs have been proposed by some research groups to have good alkali-resistant property. In their researches, five-membered heterocyclic imidazolium salts form a kind of π conjugated structure.21–23 The π conjugated structure is benefit to delocalize positive charges and hinder nucleophilic attack by hydroxyl ions through Hofmann or SN2 elimination. For example, Qiu prepared the imidazolium and QA functionalized AEMs containing styrene and acrylonitrile components.24,25 The imidazolium functionalized membranes exhibited a great electrochemical performance without visible reduction of ionic conductivity up to 1000 h, whereas the QA-functionalized membranes degraded in high pH solution. However, Varcoe et al. drawn a conflicting conclusion that the imidazolium-functionalized poly(ethylene-co-tetrafluoroethylene) showed much lower alkaline stability than QA-functionalized polymer in alkaline conditions.26–28 But they also recommend in this literature that imidazolium-type anion exchange polymer electrolytes associated with weakly alkaline carbonate/bicarbonate counteranions could be stable and be explored further for the applications where the alkalinity is relatively low. Additionally, it has been reported that imidazolium functionalized AEMs exhibited better thermal properties than the traditional QA functionalized AEMs.16,29,30 Comparing with QA analogues, imidazolium functionalized polymer electrolytes also show different solubility properties and can be dissolved in common solvents, thus permitting them to be used as catalyst layer binder to improve the ionic contact for membrane electrode assembly (MEA).
In this work, we report a new route to prepare photo-chemically cross-linkable imidazolium functionalized AEMs using poly(arylene ether ketone) (PAEK) as the base material. PAEK is a well-known high performance polymer, which has great mechanical properties, good thermal and chemical stabilities, but still low cost, and been frequently used for fabrication of ion exchange membranes.31–33 Firstly, we attached benzyl bromide moieties onto the benzyl positions of tetramethyl-containing PAEK using N-bromosuccimide (NBS) and benzoyl peroxide (BPO) through a typical free-radical substitution reaction, thus avoiding the conventional chloromethylation reaction. Secondly, bromomethylated PAEK was reacted with 1-vinylimidazole (VIm), producing imidazolium side chains that impart the resulted polymer with ionic exchange capability. The reactive unsaturated double bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) can be covalently cross-linked by the UV irradiation or heat. Finally, the VIm-functionalized PAEK was then cast for the membrane formation. The following UV-light-induced cross-linking reactions were achieved via radicals of photo-initiator activation. UV irradiation-induced cross-linking is expected to reinforce the dimensional stability and solvent resistance of imidazolium functionalized AEMs. Covalently cross-linking by adding multi (di-, tri-, or tetra-)-functional groups-containing cross-linkers has been used for QA AEMs modification by many groups.34–36 However, it usually took two or more reaction-steps for the cross-linking agent to achieve a cross-linking, and would make the process more intricate and less controllable. Compared to the covalently cross-linking, the UV-crosslinking method we reported in the present work could be simple and efficient. By adjustment of irradiation time, a series of cross-linked AEMs were obtained. The ionic conductivity, thermal and mechanical stabilities, water uptake and membrane swelling ratio were then compared in detail to investigate the influence of UV cross-linking. Moreover, the alkaline stability test of VIm-functionalized membranes under both high and relatively low alkaline conditions was carried out.
C) can be covalently cross-linked by the UV irradiation or heat. Finally, the VIm-functionalized PAEK was then cast for the membrane formation. The following UV-light-induced cross-linking reactions were achieved via radicals of photo-initiator activation. UV irradiation-induced cross-linking is expected to reinforce the dimensional stability and solvent resistance of imidazolium functionalized AEMs. Covalently cross-linking by adding multi (di-, tri-, or tetra-)-functional groups-containing cross-linkers has been used for QA AEMs modification by many groups.34–36 However, it usually took two or more reaction-steps for the cross-linking agent to achieve a cross-linking, and would make the process more intricate and less controllable. Compared to the covalently cross-linking, the UV-crosslinking method we reported in the present work could be simple and efficient. By adjustment of irradiation time, a series of cross-linked AEMs were obtained. The ionic conductivity, thermal and mechanical stabilities, water uptake and membrane swelling ratio were then compared in detail to investigate the influence of UV cross-linking. Moreover, the alkaline stability test of VIm-functionalized membranes under both high and relatively low alkaline conditions was carried out.
Experimental
Materials
3,3′,5,5′-Tetramethyl-4,4′-biphenol (TMBP) was obtained from Shanghai Jiachen chemical company and dried at 60 °C for 48 h prior to use. 4,4′-Difluorobenzophenone (DFBP) was obtained from Yanbian Longjing chemical company. NBS and BPO were purchased from Sinopharm Chemical Reagent Co. Ltd. 1-Vinylimidazole was obtained from J&K Scientific Ltd. 2,2′-Dimethoxy-2-phenylacetophenone (DMPA) was purchased from Aladdin Chemistry Co. Ltd. All other solvents and reagents were provided from Tianjin Tiantai chemical company and used without further treatment.Synthesis and bromomethylation of poly(arylene ether ketone)
Tetramethyl-containing poly(arylene ether ketone) was synthesized as follows: a 250 mL four-necked flask was prepared with a mechanical stirrer, a reflux condenser, a Dean-Stark apparatus and a thermometer. The whole device was ventilated with nitrogen for 0.5 h to drain the air. Then TMBP (9.68 g, 0.04 mol), DFBP (8.72 g, 0.04 mol), anhydrous potassium carbonate (5.52 g, 0.04 mol), dimethyl sulfoxide (DMSO, 60 mL) and toluene (20 mL) were added to the flask. First, the flask was heated to 140 °C and kept methylbenzene refluxing for 4 h. Secondly, the temperature was raised to 180 °C to remove toluene. Eight hours later, the mixture was getting more viscous and was precipitated into distilled water. Finally, the precipitates were washed by boiling water for more than ten times and dried under vacuum at 100 °C for 36 h.The bromomethylation of PAEK was shown in Scheme 1. This free-radical substitution reaction, using NBS and BPO as the brominating reagent and the initiator, was different from the usual chloromethylation reaction. The process was mild and controllable, which is described detailed as follows: the powder of PAEK (8.40 g, 0.02 mol) was dissolved in dried 1,1,2,2-tetrachloroethane in a 150 mL flask. The orange polymer solution, using nitrogen as shielding gas, was stirred by a mechanical agitator. Then NBS (5.70 g, 0.032 mol) and BPO (0.78 g, 0.0032 mol) were added into the three-necked flask. Subsequently, the mixture was hearted to the reflux temperature of 1,1,2,2-tetrachloroethane and turned to claret-red gradually. After 6 h, the claret-red mixture was precipitated out the yellow brominated polymer in ethanol. Finally, the BrPAEK polymer was obtained after dried the washed precipitate in a vacuum oven at 80 °C for 12 h.
 | ||
| Scheme 1 The bromomethylated of poly(arylene ether ketone) and the synthesis of imidazolium poly(arylene ether ketone). | ||
Synthesis of cross-linkable imidazolium functionalized poly(arylene ether ketone)
The synthesis procedure of cross-linkable imidazolium functionalized poly(arylene ether ketone) is described as follows: A 10 wt% solution of BrPAEK (4.00 g) was obtained in a flask by using N-methyl-2-pyrrolidinone (NMP) as the solvent. Then 1-vinylimidazolium (VIm, 1.20 mL) was added into the BrPAEK solution, and the container was sealed. The temperature of the flask was raised to 70 °C and held for 12 h. The mixture was precipitated in methanol and collected by filtration. After carefully washed for five times and dried at 70 °C to get rid of the methanol, a brown polymer powder (VImPAEK) was obtained.Preparation of cross-linked anion exchange membranes
Taking the VImPAEK-10 min membrane as an example: VImPAEK polymer (1.0 g) was dissolved in 10 mL NMP, and DMPA (0.05 g, 5 wt%) as the photo-initiator was added later. The solution was kept stirring for an hour to yield a stable dispersion. Then the mixture was cast onto a flat glass plate and thermally treated under vacuum at 50 °C for 24 h to get rid of the residue solvent. Subsequently, the polymer membrane attached on the glass plate was irradiated with 365 nm UV light for 10 minutes to induce cross-linking reaction. The membrane was then removed from the glass plate and soaked in 0.5 M NaHCO3 or 1 M NaOH solution for 24 h at 25 °C. After rinsed with purified water for eight times to remove extra ions, the bicarbonate (HCO3−) or hydroxide (OH−) counterion form membranes were obtained. Moreover, the thickness of all the membranes was in the range of 60–80 μm.Characterization and measurements
Chemical structure, thermal and mechanical properties of the membranes
To analyze the chemical structures of the BrPAEK and VImPAEK, 1H NMR spectra (in CDCl3 or DMSO-d6) were performed on a Bruker 510 spectrometer (500 MHz) with tetramethylsilane (TMS) as the internal standard. FT-IR spectra of powder samples were recorded between 4000 cm−1 and 500 cm−1 on a Bruker Vector 22 FT-IR spectrometer. The thermal properties of the membranes were estimated by using a Pyris 1 TGA (Perkin-Elmer) with the controllable heating rate of 10 °C min−1 in the range of 50–700 °C. All the samples were heated at 100 °C in vacuum for 2 h before measurement to remove water. The dynamic mechanical analysis (DMA) was conducted on tension film in tensile mode at a fixed frequency of 1 Hz using a TA DMA Q800. The size of the sample was 6 mm × 4 cm. The applied pre-force and oscillation amplitude were set as 1 N and 10 μm, respectively. A SHIMADZU AG-I 1 kN instrument was employed to assess the mechanical properties of VImPAEK membranes at a constant speed of 2 mm min−1. All the samples were preconditioned as 4 mm width and 15 mm length prior to measurement. At least ten samples of each type of membrane were tested to reach an average value.Water uptake and swelling ratio
The membranes were dried in vacuum oven at 100 °C for 12 h and then weighed, and the thicknesses were measured. All samples were subsequently placed into purified water at 25 °C for 48 h. After quickly wiped off the excess water on the surface, the membranes were measured to determine their weights and thicknesses immediately. The water uptake was calculated with the following equation:
 | (1) |
The swelling ratio was calculated from the change of membrane thickness by:
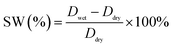 | (2) |
Ionic conductivity
The ionic conductivity of the cross-linked membranes was measured with a Princeton Applied Research Model 2273 potentiostat/galvanostat/FRA, frequency ranging from 1 MHz to 0.1 Hz, using a four-probe AC impedance method.37 Prior to the measurements, membranes were equilibrated in deionized water for 48 hours with changing the water several times. The membranes were sandwiched in a four-point cell, made from two Teflon plates and four copper strips. The samples were placed in a thermo-controlled water bath for measurement. Repeated measurements were performed at a given temperature from 30 °C to 70 °C until no more changes in the measured resistance. The ionic conductivity, σ, was determined by:
 | (3) |
Gel fraction of the cross-linked membranes
The gel fraction of the cross-linked membranes was estimated by solvent extraction.38,39 The membranes were extracted using a Soxhlet extractor in DMSO to remove those soluble components from the polymer cross-linking network. The residual fractions were vacuum-dried to a constant mass. The gel fraction was calculated by the following formula:
 | (4) |
Alkaline stability
The chemical stability of the AEMs under different alkaline conditions was evaluated by two methods: (a) the membrane samples were soaking into a strong alkaline solution (1 M NaOH) for 24 h to test whether the ionic conductivity of the membrane dropped with the testing time. (b) The membrane samples were soaking into a moderate alkaline solution (0.5 M NaHCO3) for 30 days to test the change of ionic conductivity of the membranes.Results and discussion
Structure characterization
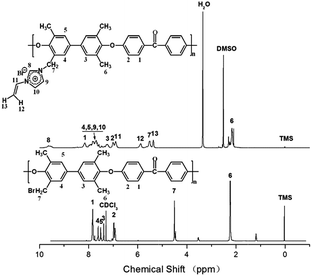
Scheme 1 shows the synthetic procedure of tetramethyl-containing poly(arylene ether ketone) and bromomethylated poly(arylene ether ketone)s. Tetramethyl-containing poly(arylene ether ketone) was synthesized according to our previously report.9 The conversion ratio of methyl groups to bromomethyl groups could be controlled by adjusting the amount of NBS used. The number of the bromine groups per repeat unit (DBr), which was defined as the ratio of bromomethyl groups converted from methyl groups, can be calculated from their 1H NMR spectra. As shown in Fig. 1, the peaks around δ 2.24 ppm (H6) assigned to the methyl protons and the typical peaks observed at δ 4.40 ppm (H7) correspond to the brominated methylene protons. As for the resulting BrPAEK, the DBr was determined to be 1.56 by comparing the integration ratio of H6 to H7, demonstrating that BrPAEK with approximate 1.56 benzyl bromine atoms per repeat unit was obtained.The synthesis of photosensitive VImPAEK was carried out under a very mild condition as shown in Scheme 1. The reaction of converting benzyl bromide groups to vinylimidazolium groups was almost complete. For the 1H NMR spectrum of VImPAEK in Fig. 1, the new peaks appeared at δ 5.34 ppm (H13), δ 5.47 ppm (H7), δ 5.79 ppm (H12) and δ 9.42 ppm (H8), which are ascribed to the protons of the vinylimidazolium cations. After the quaternization of BrPAEK with 1-vinylimidazole, the signals at δ 4.40 ppm for the bromomethylenes of BrPAEK disappeared in the spectrum of VImPAEK, indicating nearly complete conversion of bromomethyl groups to the imidazolium groups.
DMPA and the main UV crosslinking mechanism of VImPAEK
The photo-crosslinking using UV or visible light to increase the mechanical strengths and to enhance the chemical and thermal stabilities of proton exchange membranes has been extensively investigated by many research groups.40–42 Here, we firstly applied the photo-crosslinking technology to improve the performance of an AEM for applications in alkaline electrochemical devices. To fulfil an efficient and extensive cross-linking, the photo-initiator is a critical factor. DMPA is one kind of benzoic alkyl ether photo-initiators, which has a high photo-initiation activity in initiating double bond cross-linking. As shown in Scheme 2, a DMPA molecular was split into two reactive free radicals under UV radiation. Actually both kinds of free radicals have the photo-initiation activity. The experimental procedure was as follows: DMPA was added into VImPAEK solution and well dispersed under vigorously stirring. After a vacuum-dried process at 80 °C, the VImPAEK membranes were radiated for a few minutes. For example, VImPAEK-10 min indicated the membrane was kept 10 minutes under irradiation with 365 nm UV light. The peel-off cross-linked VImPAEK membranes were then transformed to OH− forms (VImPAEK-OH) by 1 M NaOH solution or HCO3− forms (VImPAEK-HCO3) by 0.5 M NaHCO3 for the further investigation.Gel fraction of the cross-linked membranes
As shown in Fig. 2, the photocross-linked membranes could not be dissolved in any common solvents after exposing to UV irradiation. The gel fraction evaluated by solution extraction revealed the cross-linking levels. The values of gel fraction are 36.01% (VImPAEK-5min), 41.57% (VImPAEK-10 min) and 51.56% (VImPAEK-15 min). The increasing gel fraction could be due to the longer irradiation time which activated much more amounts of radicals, thus resulting in the higher density of the cross-linking.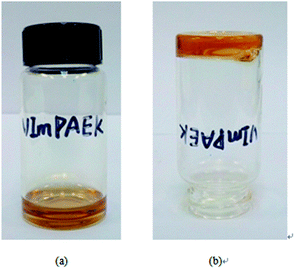 | ||
| Fig. 2 The photograph for (a) the status of VImPAEK before UV-irradiation and (b) the status of VImPAEK after UV-irradiation. | ||
Thermal gravimetric analysis and dynamic mechanical analysis
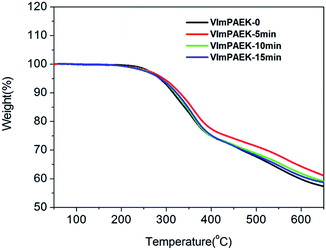
The TGA curves of VImPAEK membranes are shown in Fig. 3. There are two drastic weight losses as the temperature increased from 50 °C to 650 °C under nitrogen atmosphere. The temperature of the first weight loss corresponding to the decomposition of vinylimidazolium groups was around 240 °C, and the second decomposition stage starting from 450 °C was due to the degradation of the main polymer chain. Because of the cross-linking with UV irradiation, the decomposition onset temperature for the first weight loss of the cross-linked membranes, including VImPAEK-5min, VImPAEK-10 min and VImPAEK-15 min are slightly higher than that of uncross-linked VImPAEK-0, indicating a better thermal stability of the cross-linked samples than the pristine one. All these VImPAEK membranes exhibited no tiny weight loss lower than 220 °C, indicating all VImPAEK membranes had a high heat-resistant temperature for the potential applications as AEMs.The glass transition temperature (Tg) of VImPAEK membranes was measured by DMA and Tg values derived from tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ are shown in Fig. 4. A slight increasing tendency was observed in the Tg values of UV-cross-linked membranes as the UV irradiation time increased from 5 to 15 min, which was higher than that of the uncross-linked VImPAEK-0. The glass transition temperature of polymer can be affected by many factors, for instance, the structure of polymer chain (including main chain and pendant group) and the intermolecular force. For the VImPAEK membranes, the structure of main chain was totally same. After the photo-crosslinking treatment, a part of pendant vinylimidazolium groups connected with each other, which increased the steric hindrance of intermolecular rotation and led to the higher Tg. The VImPAEK-15 min with the longest irradiation time had the highest Tg (251 °C) due to the increasing amounts of cross-linking sites. However, due to the limited crosslinking degree, which can be inferred from the gel fraction (36.01–51.56%), there was no obvious change in Tg values for these photo-crosslinked VImPAEK membranes.
δ are shown in Fig. 4. A slight increasing tendency was observed in the Tg values of UV-cross-linked membranes as the UV irradiation time increased from 5 to 15 min, which was higher than that of the uncross-linked VImPAEK-0. The glass transition temperature of polymer can be affected by many factors, for instance, the structure of polymer chain (including main chain and pendant group) and the intermolecular force. For the VImPAEK membranes, the structure of main chain was totally same. After the photo-crosslinking treatment, a part of pendant vinylimidazolium groups connected with each other, which increased the steric hindrance of intermolecular rotation and led to the higher Tg. The VImPAEK-15 min with the longest irradiation time had the highest Tg (251 °C) due to the increasing amounts of cross-linking sites. However, due to the limited crosslinking degree, which can be inferred from the gel fraction (36.01–51.56%), there was no obvious change in Tg values for these photo-crosslinked VImPAEK membranes.
Mechanical properties
The mechanical properties of VImPAEK membranes were measured and their stress–strain curves are shown in Fig. 5. The uncross-linked VImPAEK-0 exhibited a Young's modulus of 1.01 GPa, a tensile strength at maximum load of 49.17 MPa, and an elongation at break of 16.8%. The UV-cross-linked VImPAEK membranes, showed much higher tensile strength and Young's modulus, whereas smaller elongation at break than those of pristine VImPAEK-0 membrane. The enhancement in the mechanical strength of the cross-linked membranes could be ascribed to the enhanced intermolecular force and the compact cross-linking structure under UV irradiation treatment. The reduction of elongation at break was attributed to the confined mobility of the polymer chain because of increasing cross-linking density. Furthermore, the Young's modulus and tensile strength of cross-linked membranes were in the order of VImPAEK-15 min (1.99 GPa, 61.38 MPa) > VImPAEK-10 min (1.71 GPa, 60.48 MPa) > VImPAEK-5 min (1.53 GPa, 53.63 MPa). It was evident that the photocross-linked membranes could be gradually reinforced by increasing the UV irradiation time and increasing the cross-linked network density.Water uptake and swelling ratio
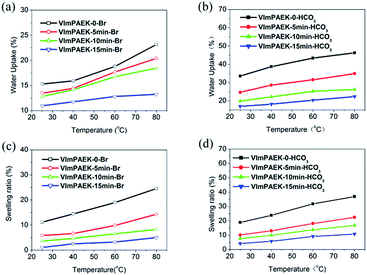
The water uptake and swelling ratio of both the VImPAEK membranes in Br− forms (VImPAEK-XX-Br) and VImPAEK membranes in HCO3− forms (VImPAEK-XX-HCO3) were investigated as a function of temperature. The water uptake affects the conductivity of the membrane in some ways, whereas an ultra water uptake causes undesired changes in dimensional stabilities and a loss in mechanical properties. Fig. 6 showed that the water uptake and swelling ratio of all the membranes increased with the temperature increasing spontaneously. It could be attributed to the improve mobility of polymer molecules and higher free volume for water uptake at a higher temperature. The cross-linked membranes showed lower water uptake as the UV irradiation time increased. For example, the uncross-linked VImPAEK-0-HCO3 exhibited a water uptake of 46.25% at 80 °C. During the 15 minutes of UV irradiation, the water uptake of VImPAEK-15 min-HCO3 decreased to 22.29%. The rigid 3-dimensional cross-linked network structure restricts the motion of polymer chains, and hindered the ionic domains to aggregate to form continuous ionic conducting channels, thus resulting in the decreasing water uptake. Additionally, the water uptake values of VImPAEK-XX-HCO3 associated with the bicarbonate counter-anion were approximately 2 times higher than those of the corresponding VImPAEK-XX-Br. The difference in water uptake is likely due to the difference in the alkalinity between Br− and HCO3− in the membranes.The swelling ratio of these VImPAEK membranes showed a similar tendency to water uptake with increasing the UV irradiation time. The un-crosslinked VImPAEK-0-HCO3 exhibited a swelling ratio of 36.91% (80 °C), while the cross-linked VImPAEK-15 min-HCO3 showed a swelling ratio of 10.74%, which is one third lower than that of the value of VImPAEK-0-HCO3. The results indicate that the photocross-linking technique is an efficient approach to prevent unacceptable dimensional changes of the hydrated membranes, especially at higher operating temperatures. This is also the primary purpose for us to utilize the cross-linking treatment.
Ionic conductivity
For the ion exchange membrane, ionic conductivity is a key property to achieve its function. All the VImPAEK-XX-HCO3 membranes before and after cross-linking treatment were measured under 100% RH. Fig. 7 showed the ionic conductivity of all the membranes changed as a function of temperature. It is obvious that the ionic conductivity values increased with the temperature increasing. As expected, they exhibited the highest ionic conductivities ranging from 0.021 S cm−1 to 0.035 S cm−1 at 70 °C. The ionic conductivity of cross-linked VImPAEK membranes decreased with the UV irradiation time as compared with that of the pristine membrane. The decreasing tendency is similar to the water uptake, indicating that the reduction of conductivity mostly arose from the loss of water uptake after cross-linking treatment. With increasing the irradiation time, the cross-linked structure of VImPAEK-15 min-HCO3 was more compact than that of VImPAEK-10 min-HCO3 or VImPAEK-5 min-HCO3, thus leading to a relatively low conductivity. It should be noted that the cross-linking reaction only occurred on the double bonds of the vinylimidazole groups in the side chains, which not consumed the functional groups. The ionic conductivity of cross-linked membranes is still acceptable for the conduction application. Furthermore, the optimum performance of VImPAEK membranes could be controlled by adjusting the photo-irradiation time. For example, the VImPAEK-15 min-HCO3 membrane with photo-irradiation time of 15 min showed the moderate ionic conductivity of 0.021 S cm−1 and a low swelling ratio of 10.74% at 70 °C. It also has the highest Young's modulus of 1.99 GPa and tensile strength of 61.38 MPa. Therefore, it is possible to achieve a relative balance of the ionic conductivity and the membrane swelling by controlling the photo-irradiation time. | ||
| Fig. 7 The ionic conductivities of VImPAEK membranes in bicarbonate form at different photo-irradiation time. | ||
Ionic diffusion rates of membranes
Using the Nernst–Einstein equation shown below,43,44 the corresponding ionic diffusion coefficients D can be estimated to explain the changing trend in ionic conductivities of VImPAEK membranes.
 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), R is the gas constant (8.314 J mol−1 K−1) and T is the temperature (K). As shown in Table 1, ionic diffusion coefficients in the membrane increased with the improved temperature. The ionic diffusion coefficients at 60 °C are as almost two times as the corresponding values at 40 °C. The ionic diffusion coefficient of pristine membrane VImPAEK-0-HCO3 is higher than that of cross-linked ones, and ionic diffusion coefficients decrease with the increase of UV irradiation time for the cross-linked membranes. The reduction may be derived from the loss of water uptake and more compact structure. Anions in cross-linked VImPAEK-HCO3 membranes diffuse more slowly, resulting in the decline of ionic conductivity.
485 C mol−1), R is the gas constant (8.314 J mol−1 K−1) and T is the temperature (K). As shown in Table 1, ionic diffusion coefficients in the membrane increased with the improved temperature. The ionic diffusion coefficients at 60 °C are as almost two times as the corresponding values at 40 °C. The ionic diffusion coefficient of pristine membrane VImPAEK-0-HCO3 is higher than that of cross-linked ones, and ionic diffusion coefficients decrease with the increase of UV irradiation time for the cross-linked membranes. The reduction may be derived from the loss of water uptake and more compact structure. Anions in cross-linked VImPAEK-HCO3 membranes diffuse more slowly, resulting in the decline of ionic conductivity.
| Samples | Ionic diffusion coefficient (cm2 s−1, 40 °C) | Ionic diffusion coefficient (cm2 s−1, 60 °C) |
|---|---|---|
| VImPAEK-0-HCO3 | 6.41 × 10−7 | 1.24 × 10−6 |
| VImPAEK-5 min-HCO3 | 3.41 × 10−7 | 7.00 × 10−7 |
| VImPAEK-10 min-HCO3 | 2.52 × 10−7 | 4.76 × 10−7 |
| VImPAEK-15 min-HCO3 | 1.60 × 10−7 | 3.06 × 10−7 |
Alkaline stability
To study the stability of the VImPAEK membranes under stronger alkaline conditions, pieces of the membranes were soaked in 1 M NaOH aqueous solution. After immersing these membranes into NaOH solution, they quickly turned brownish yellow in color, suggesting a possible chemical change in strong alkaline conditions. The hydroxide conductivities of VImPAEK membranes in hydroxide form after soaking in alkaline solution for 24 h and in Br− form before soaking are displayed in Fig. 8. The ionic conductivities of the cross-linked VImPAEK-10 min and uncross-linked VImPAEK-0 membranes in OH− form are close to the values of them in original Br− form, and much lower than those in HCO3− form. The observations here do not agree with those reported for imidazolium AEMs.45,46 In their reports, the OH− conductivities were much higher than the HCO3− and Br− conductivities, and the prediction according to the diluted solution mobility ratio of the anions showed the conductivity of OH− is about 4.4 times than that of HCO3−. The lower OH− conductivity implied that imidazolium cations had been degraded for VImPAEK membranes in 1 M NaOH no matter if the membranes were cross-linked or not. Despite its compact structure, the cross-linking density has little steric hindrance for the imidazolium cations to be attacked by hydroxyl ions in stronger alkaline environment.Ye et al. used 1HNMR to determine the chemical stability of the imidazolium cation where an aldehyde group was observed in the spectrum of degraded membrane.46 They proposed that the imidazolium cation would be degraded via a ring-opening mechanism which is triggered by the nucleophilic attack of OH− on the imidazolium ring at C2 position. According to their work, the possible procedure of ring-opening for the vinylimidazolium cation of VImPAEK membrane could be illustrated in Scheme 3. We also tried to use NMR analysis to confirm the illustrated mechanism of VImPAEK membranes. Unfortunately, even the pristine uncrosslinked VImPAEK-0 membrane was insoluble in common solvents after immersing in NaOH solution for 24 h, indicating that a self-crosslinking occurred during the alkaline treatment. Therefore, instead of 1H NMR measurement, we applied FTIR to determine the alkaline stability of the vinylimidazolium cation. Fig. 9 shows the FTIR spectra of VImPAEK membranes before and after immersing in 1 M NaOH solution for 24 h. The membrane used before immersion was in original Br− form. After immersion test, the intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N band on the vinylimidazolium ring existing at 1572 cm−1 and 1551 cm−1 decreased to a great extent, indicating the mass degradation of vinylimidazolium cation.
N band on the vinylimidazolium ring existing at 1572 cm−1 and 1551 cm−1 decreased to a great extent, indicating the mass degradation of vinylimidazolium cation.
 | ||
| Fig. 9 The FTIR spectra of VImPAEK membranes (a) before and (b) after immersing in 1 M NaOH solution for 24 h. | ||
Although the vinylimidazolium cation was unstable in strong alkali solution, they were rather stable in weak base, such as NaHCO3. We set a condition of 0.5 M NaHCO3 at 60 °C for 30 days to evaluate the stability of vinylimidazolium cations. As displayed in Fig. 10, there is no visible change in the ionic conductivity of VImPAEK-10 min-HCO3− during the stability test. The ionic conductivity was maintained at a constant value of 0.017 S cm−1 after 30 days. It proved that the cross-linked vinylimidazolium-based membranes are stable in the bicarbonate anion form and there is no degradation of vinylimidazolium groups under relatively low alkaline conditions. Therefore, we believe that these UV-cross-linked membranes in bicarbonate form are allowed to apply in electrochemical devices that are operated at less extreme pHs, such as reverse electrodialysis cells.47–49
 | ||
| Fig. 10 The ionic conductivities of the VImPAEK-10 min membrane in bicarbonate form after soaking in 0.5 M NaHCO3 at 60 °C. | ||
Conclusions
A novel photo-crosslinkable vinylimidazolium-functionalized poly (arylene ether ketone) was obtained by quaternization of bromometylated PAEK with 1-vinylimidazole and subsequent anion exchange reaction. Then, a new strategy to improve the performances of photo-crosslinking AEMs by was demonstrated. The UV-irradiation-induced cross-linking occurred on the pendant vinylimidazolium cations realized the desired effects of decreasing swelling ratios in water, improving the dimensional, thermal and mechanical stabilities of AEMs, and the HCO3− conductivity was still acceptable. VImPAEK-15 min-HCO3 membrane with photo-irradiation 15 min showed the ionic conductivity of 0.021 S cm−1 with a low swelling ratio of 10.74% at 70 °C. It also has the highest Young's modulus of 1.99 GPa and tensile strength of 61.38 MPa. The alkaline stability test showed that the cross-linked membranes are chemical stable in the bicarbonate anion form under mild alkaline conditions, which indicated that they could be used in electrochemical devices operating at less extreme pHs. All these results indicated that the photo-crosslinking could be an easy and effective method to enhance the membrane properties. Therefore, the UV-crosslinking strategy could be widely utilized to balance the contradiction between dimensional stability and ionic conductivity of alkaline polymer electrolytes.Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant No. 21474036 and 21374034), Science and Technology Development Plan of Jilin Province (Grant No. 20130522138JH) and the Fok Ying-Tong Education Foundation for Young Teachers in the Higher Education Institutions of China (Grant No. 142010).Notes and references
- N. J. Robertson, H. A. Kostalik, T. J. Clark, P. F. Mutolo, H. D. Abruna and G. W. Coates, J. Am. Chem. Soc., 2010, 132, 3400–3404 CrossRef CAS PubMed.
- S. F. Lu, J. Pan, A. B. Huang, L. Zhuang and J. T. Lu, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 20611–20614 CrossRef CAS.
- B. Schwenzer, J. L. Zhang, S. Kim, L. Y. Li, J. Liu and Z. G. Yang, ChemSusChem, 2011, 4, 1388–1406 CrossRef CAS PubMed.
- W. Garcia-Vasquez, L. Dammak, C. Larchet, V. Nikonenko, N. Pismenskaya and D. Grande, J. Membr. Sci., 2013, 446, 255–265 CrossRef CAS PubMed.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J. M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- J. Wang, Z. Zhao, F. Gong, S. Li and S. Zhang, Macromolecules, 2009, 42, 8711–8717 CrossRef CAS.
- S. Xu, G. Zhang, Y. Zhang, C. Zhao, W. Ma, H. Sun, N. Zhang, L. Zhang, H. Jiang and H. Na, J. Power Sources, 2012, 209, 228–235 CrossRef CAS PubMed.
- J. Pan, C. Chen, L. Zhuang and J. Lu, Acc. Chem. Res., 2011, 45, 473–481 CrossRef PubMed.
- W. Ma, C. Zhao, H. Lin, G. Zhang and H. Na, J. Appl. Polym. Sci., 2011, 120, 3477–3483 CrossRef CAS PubMed.
- N. W. Li, Y. J. Leng, M. A. Hickner and C. Y. Wang, J. Am. Chem. Soc., 2013, 135, 10124–10133 CrossRef CAS PubMed.
- J. Ran, L. Wu and T. W. Xu, Polym. Chem., 2013, 4, 4612–4620 RSC.
- R. Janarthanan, S. Kishore Pilli, J. L. Horan, D. A. Gamarra, M. R. Hibbs and A. M. Herring, J. Electrochem. Soc., 2014, 161, F944–F950 CrossRef CAS PubMed.
- T. C. M. Chung, Macromolecules, 2013, 46, 6671–6698 CrossRef CAS.
- S. Chempath, J. M. Boncella, L. R. Pratt, N. Henson and B. S. Pivovar, J. Phys. Chem. C, 2010, 114, 11977–11983 CAS.
- S. Chempath, B. R. Einsla, L. R. Pratt, C. S. Macomber, J. M. Boncella, J. A. Rau and B. S. Pivovar, J. Phys. Chem. C, 2008, 112, 3179–3182 CAS.
- M. Guo, J. Fang, H. Xu, W. Li, X. Lu, C. Lan and K. Li, J. Membr. Sci., 2010, 362, 97–104 CrossRef CAS PubMed.
- Y. Ye, K. K. Stokes, F. L. Beyer and Y. A. Elabd, J. Membr. Sci., 2013, 443, 93–99 CrossRef CAS PubMed.
- Q. Zhang, S. Li and S. Zhang, Chem. Commun., 2010, 46, 7495–7497 RSC.
- X. Lin, L. Wu, Y. Liu, A. L. Ong, S. D. Poynton, J. R. Varcoe and T. Xu, J. Power Sources, 2012, 217, 373–380 CrossRef CAS PubMed.
- J. Wang, G. He, X. Wu, X. Yan, Y. Zhang, Y. Wang and L. Du, J. Membr. Sci., 2014, 459, 86–95 CrossRef CAS PubMed.
- B. Lin, H. Dong, Y. Li, Z. Si, F. Gu and F. Yan, Chem. Mater., 2013, 25, 1858–1867 CrossRef CAS.
- B. Lin, L. Qiu, J. Lu and F. Yan, Chem. Mater., 2010, 22, 6718–6725 CrossRef CAS.
- W. Li, J. Fang, M. Lv, C. Chen, X. Chi, Y. Yang and Y. Zhang, J. Mater. Chem., 2011, 21, 11340 RSC.
- B. Qiu, B. Lin, L. Qiu and F. Yan, J. Mater. Chem., 2012, 22, 1040 RSC.
- B. Qiu, B. Lin, Z. Si, L. Qiu, F. Chu, J. Zhao and F. Yan, J. Power Sources, 2012, 217, 329–335 CrossRef CAS PubMed.
- O. I. Deavin, S. Murphy, A. L. Ong, S. D. Poynton, R. Zeng, H. Herman and J. R. Varcoe, Energy Environ. Sci., 2012, 5, 8584 CAS.
- X. Lin, J. R. Varcoe, S. D. Poynton, X. Liang, A. L. Ong, J. Ran, Y. Li and T. Xu, J. Mater. Chem. A, 2013, 1, 7262 CAS.
- O. M. M. Page, S. D. Poynton, S. Murphy, A. Lien Ong, D. M. Hillman, C. A. Hancock, M. G. Hale, D. C. Apperley and J. R. Varcoe, RSC Adv., 2013, 3, 579 RSC.
- F. Zhang, H. Zhang and C. Qu, J. Mater. Chem., 2011, 21, 12744 RSC.
- A. H. N. Rao, R. L. Thankamony, H.-J. Kim, S. Nam and T.-H. Kim, Polymer, 2013, 54, 111–119 CrossRef CAS PubMed.
- M.-H. Jeong, K.-S. Lee, Y.-T. Hong and J.-S. Lee, J. Membr. Sci., 2008, 314, 212–220 CrossRef CAS PubMed.
- H. Lin, C. Zhao, Z. Cui, W. Ma, T. Fu, H. Na and W. Xing, J. Power Sources, 2009, 193, 507–514 CrossRef CAS PubMed.
- J. Pang, S. Feng, Y. Yu, H. Zhang and Z. Jiang, Polym. Chem., 2014, 5, 1477 RSC.
- A. Katzfuß, S. Poynton, J. Varcoe, V. Gogel, U. Storr and J. Kerres, J. Membr. Sci., 2014, 465, 129–137 CrossRef PubMed.
- S. Zhang, C. Li, X. Xie and F. Zhang, Int. J. Hydrogen Energy, 2014, 39, 13718–13724 CrossRef CAS PubMed.
- Z. Liu, X. L. Zhu, G. F. Wang, X. X. Hou and D. Z. Liu, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 1632–1638 CAS.
- S. Slade, S. A. Campbell, T. R. Ralph and F. C. Walsh, J. Electrochem. Soc., 2002, 149, A1556 CrossRef CAS PubMed.
- J. Li, L. Zhang, J. Gu, Y. Sun and X. Ji, RSC Adv., 2015, 5, 19859–19864 RSC.
- H. Pan, Y. Zhang, H. Pu and Z. Chang, J. Power Sources, 2014, 263, 195–202 CrossRef CAS PubMed.
- H. Liu, M.-H. Lee and J. Lee, Macromol. Res., 2009, 17, 725–728 CrossRef CAS.
- S. Zhong, C. Liu and H. Na, J. Membr. Sci., 2009, 326, 400–407 CrossRef CAS PubMed.
- P. Wen, Z. Zhong, L. Li, F. Shen, X.-D. Li and M.-H. Lee, J. Membr. Sci., 2014, 463, 58–64 CrossRef CAS PubMed.
- M. L. Di Vona, D. Marani, A. D'Epifanio, S. Licoccia, I. Beurroies, R. Denoyel and P. Knauth, J. Membr. Sci., 2007, 304, 76–81 CrossRef CAS PubMed.
- Y. Liu, J. Wang, Y. Yang, T. M. Brenner, S. Seifert, Y. Yan, M. W. Liberatore and A. M. Herring, J. Phys. Chem. C, 2014, 118, 15136–15145 CAS.
- D. Chen and M. A. Hickner, ACS Appl. Mater. Interfaces, 2012, 4, 5775–5781 CAS.
- Y. Ye and Y. A. Elabd, Macromolecules, 2011, 44, 8494–8503 CrossRef CAS.
- J. R. Varcoe, P. Atanassov, D. R. Dekel, A. M. Herring, M. A. Hickner, P. A. Kohl, A. R. Kucernak, W. E. Mustain, K. Nijmeijer, K. Scott, T. Xu and L. Zhuang, Energy Environ. Sci., 2014, 7, 3135–3191 CAS.
- M. D. Eisaman, L. Alvarado, D. Larner, P. Wang, B. Garg and K. A. Littau, Energy Environ. Sci., 2011, 4, 1319–1328 CAS.
- P. Leung, X. Li, C. P. de León, L. Berlouis, C. T. J. Low and F. C. Walsh, RSC Adv., 2012, 2, 10125 RSC.
| This journal is © The Royal Society of Chemistry 2015 |