An integrated flow and microwave approach to a broad spectrum protein kinase inhibitor†
Cecilia Russella,
Andrew J. S. Lina,
Peter Hainsb,
Michela I. Simonea,
Phillip J. Robinsonb and
Adam McCluskey*a
aCentre for Chemical Biology, Chemistry, School of Environmental and Life Science, The University of Newcastle, University Drive, Callaghan, New South Wales 2308, Australia. E-mail: Adam.McCluskey@newcastle.edu.au; Fax: +61 249 215472; Tel: +61 249 216486
bChildren's Medical Research Institute, 214 Hawkesbury Road, Westmead, NSW 2145, Australia
First published on 5th October 2015
Abstract
The protein kinase inhibitor CTx-0152960 (6, 2-((5-chloro-2-((4-morpholinophenyl)amino)pyrimidin-4-yl)amino)-N-methylbenzamide), and the piperazinyl analogue, CTx-0294885 (7, 2-((5-chloro-2-((4-piperazin-1-ylphenyl)amino)pyrimidin-4-yl)amino)-N-methylbenzamide), were prepared using a hybrid flow and microwave approach. The use of flow chemistry approaches avoided the need for Boc-protection of piperidine in the key SNAr coupling with 1-fluoro-4-nitrobenzene. Microwave coupling of 4-morphilinoaniline 8 and 4-(piperazine-1-yl)aniline 9 with 2-(2,5-dichloropyrimidine-4-ylamino)-N-methylbenzamide 10, proved to be the most efficacious route to the target analogues 6 and 7. This hybrid methodology reduced the number of synthetic steps, gave enhanced overall yields and increased atom economy through step reduction and minimal requirement for chromatographic purification, relative to the original batch synthesis approach.
Introduction
Protein kinase inhibitors (PKIs) are currently one of the most important classes of anticancer drugs. Globally there continues to be a major drive towards the development of more selective and potent PKI-based drugs for the treatment of an expanding range of diseases.1–5 Imatinib was the first clinically approved PKI in 2001, with 14 PKI-based drugs approved for the treatment of cancer over the next decade, e.g. gefitinib (2, 2002), vemurafenib (3, 2011) and ruxolitinib (4, 2011) (Fig. 1).6While there are a number of selective inhibitors, the chemical biology of protein kinases has benefited greatly from the discovery and subsequent use of the broad spectrum inhibitor staurosporine (5).7 Broad spectrum PKIs such as staurosporine, are of particular importance as chemical proteomics tools.8–16 Recently 2-(5-chloro-2-(4-morpholinophenylamino)-pyrimidin-4-ylamino)-N-methylbenzamide, CTx-0152960 (6), was reported as a new member of the broad spectrum PKI family and developed as a Sepharose-supported kinase capture reagent in the identification of 235 protein kinases from a digest of human breast adenocarcinoma, MDA-MB-231, cells (Fig. 2, 7, CTx-0294885).17,18
 | ||
| Fig. 2 2-(5-Chloro-2-(4-morpholinophenylamino)pyrimidin-4-ylamino)-N-methylbenzamide (6) and 2-(5-chloro-2-(4-(piperazine-1-yl)phenylamino)pyrimidin-4-ylamino)-N-methylbenzamide (7). | ||
Given the importance of the PKI families of compounds they have attracted considerable attention from the synthetic community, more recently in the application of flow chemistry approaches.19,20 As part of an on-going program we were interested in gaining access to these probes and the parent inhibitor compound to conduct competition based kinomics experiments.8,12,21 The synthesis of 6 and 7 was detailed by Zhang,17,18 but as we desired access to both compounds we viewed this as an opportunity to investigate the introduction of potential efficiencies in the synthesis of both compounds via a combination of flow and microwave chemistry approaches.22–29
Results and discussion
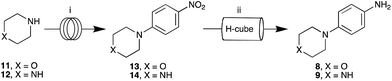
On examination of 6 and 7, we identified fragments 8/9 and 10 as key intermediates (Fig. 3). Access to piperazine 9, on coupling to 10, would facilitate direct conjugation to a solid support such as Sepharose,17 while the use of morpholine 8 would allow access to 6. We thus set about developing the required protocols. | ||
| Fig. 3 Identification of two key fragments for the synthesis of 6 and 7, the anilines 8 and 9, and the pyrimidine 10. | ||
Our initial investigations centred on the use of mono-boc piperazine. While commercially available, we were also interested in accessing an approach applicable across a myriad of diamine substrates. Despite the apparent simplicity of the synthesis, current batch approaches to mono-boc piperazine via Boc2O and tert-butyl azido formate, typically afford a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of mono- and di-Boc piperazine.30,31 This was further confounded by the explosive nature of tert-butyl azido formate.32 Despite considerable optimisation, the flow selective mono-boc protection was unsuccessful, and in keeping with a recent report by Jong and Bradley, our optimal outcome was a 88
1 ratio of mono- and di-Boc piperazine.30,31 This was further confounded by the explosive nature of tert-butyl azido formate.32 Despite considerable optimisation, the flow selective mono-boc protection was unsuccessful, and in keeping with a recent report by Jong and Bradley, our optimal outcome was a 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 ratio.33 Despite this, we were able to carry the di-boc-piperazine through all subsequent synthetic steps with no major complications (ESI†).
12 ratio.33 Despite this, we were able to carry the di-boc-piperazine through all subsequent synthetic steps with no major complications (ESI†).
Re-evaluation of our flow strategy saw omission of the protecting group step and investigation of the direct SNAr coupling of piperazine 12 with 1-fluoro-4-nitrobenzene. Optimisation of the flow SNAr coupling of 12 with 1-fluoro-4-nitrobenzene at 80 °C and 5 mL min−1 (Vapourtec R2+), using 5 equivalents of 12 gave exclusively 1-(4-nitrophenyl)piperazine 14 in a 95% yield (Scheme 1). The equivalent batch approaches furnish a mixture of mono- and di-adduct in keeping with our earlier efforts to mono-protect piperazine (ESI†).
Flow hydrogenation of 14's nitro moiety was effected cleanly as a 0.05 M MeOH solution using 10%-Pd/C at 50 °C and 50 bar H2 at 1 mL min−1 to give quantitative conversion to aniline 9 (Scheme 1; ThalesNano H-CubePro®; 70 mm CatCart®).22,25,34,35 This sequence, commencing with morpholine 11 gave the corresponding morpholino analogues 13 and 8 in excellent yields (76 and 87%, respectively) and purity (>98%) (ESI†).
Synthesis of the pyrimidine fragment 10, commenced with the treatment of isatoic anhydride 15 with 40% (w/w) aq. MeNH2 at 0.5 mL min−1 at 0 °C (Syrris FRX-100) to give, N-methylamidoaniline 16 in 79% yield (Scheme 2). The yield was identical to that obtained in the corresponding batch process. Access to pyrimidine 10 was effected by an SNAr coupling with 2,3,5-trichloropyrimidine at 100 °C and 0.25 mL min−1 (Vapourtec R2+). Pyrimidine 10 was isolated by simple filtration in 71% yield and >95% purity after concentration of the reaction stream, whereas the batch approach required longer reaction times (ESI†).17
 | ||
| Scheme 2 Reagents and conditions. (i) Syrris FRX-100: 40% w/w aq. MeNH2, 0.5 mL min−1, 0 → 19 °C, 19 h; (ii) Vapourtec R2+: 2,3,5-trichloropyrimidine, iPr2NEt, iPrOH, 4 bar, 100 °C. | ||
With fragments 8/9 and 10 in hand, we next examined the coupling to access 6 and 7. All flow chemistry approaches failed to afford a satisfactory outcome with considerable reagent degradation observed at the temperatures and residence times required to effect an acceptable level of coupling. However, microwave assisted coupling of 9 and 10 provided 7 in a 47% yield, as a mixture of benzamide rotamers.36 An identical microwave assisted coupling of 8 and 10 gave 6 in 51% yield (Scheme 3).
Conclusions
The synthesis of 13 and 14 were readily accomplished through flow SNAr reaction either morpholine 11 or piperazine 12 with 1-flouro-4-nitrobenzene, the subsequent aryl nitro functionality of 13 and 14 were reduced to the corresponding anilines 8 and 9, via flow hydrogenation. No protection of the piperazine moiety was required under these conditions. This allowed a reduction in the number of chemical steps, and thus an increase in atom economy overall. The coupling of 8/9 with 10 under flow conditions failed, but was smoothly affected under microwave irradiation.Our hybrid flow and microwave assisted route provided rapid and scalable access to CTx-0152960 (6) and CTx-0294885 (7) in a five step process in overall yields of 19 and 25%, requiring essentially filtration through a short silica gel plug with minimal chromatographic requirement. This compares favourably against the initial synthesis of 7 in 6.5% yield, with multiple chromatographic requirements.17
Experimental
All reagents were purchased from Sigma-Aldrich, Matrix Scientific or Lancaster Synthesis and were used without purification. With the exception of THF (anhydrous > 99%) obtained from Sigma-Aldrich, all solvents were re-distilled from glass prior to use.1H and 13C NMR spectra were recorded on a Brüker Avance™ AMX 400 MHz spectrometer at 400 and 101 MHz, respectively. Chemical shifts (δ) are reported in parts per million (ppm) measured relative to the internal standards, and coupling constants (J) are expressed in Hertz (Hz). Mass spectra were recorded on a Shimadzu LCMS 2010 EV using a mobile phase of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile
1 acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O with 0.1% formic acid.
H2O with 0.1% formic acid.
HPLC analysis was recorded using a Shimadzu 20A with Grace econosphere C18 5μ 150 × 4.6 mm column, and GCMS analysis was carried out using a Shimadzu QP 2010 GCMS.
4-(4-Nitrophenyl)morpholine (13)
A stream of morpholine (11) (0.436 g, 5.0 mmol) in DMF (100 mL) and another stream of 4-fluoronitrobenzene (0.352 g, 2.5 mmol) in DMF (100 mL) was passed through a Vapourtec R2+ reaction system at 0.5 mL min−1. The instrument was fitted with two 10 mL PFA coils, maintained at 120 °C and 8 bar of pressure. The resulting product stream was taken up in DCM (200 mL) and washed with water (2 × 100 mL) and saturated NaCl (1 × 100 mL). The organic layer was then dried (MgSO4) and concentrated in vacuo to give a yellow solid (0.396 g, 76% yield).1H NMR (CDCl3, 400 MHz): δ 8.13 (2H, d, J = 9.3 Hz), 6.82 (2H, d, J = 9.3 Hz), 3.61 (4H, m), 3.42 (4H, m), 1.49 (9H, s); 13C NMR (CDCl3, 101 MHz): δ 154.6, 154.6, 138.8, 126.0 (2C), 112.9 (2C), 80.4, 46.9 (2C), 43.9–42.1 (bs, 2C), 28.4 (3C); mass spectrum (ESI, +ve) 308 m/z [(M + H)+, 20%], 252 (100), 208 (7), 146 (7), 100 (13); FTIR νmax (cm−1): 3007, 2970, 2865, 167, 1585, 1485, 1417, 1367, 1318, 1238, 1162, 1112, 1040, 1081, 1001, 919, 830, 774, 754, 714, 691, 666, 640, 538, 500.
1-(4-Nitrophenyl)piperazine (14)
A stream of piperazine (12, 1.05 g, 12.1 mmol), in DMF (50 mL) solution and another stream of 4-fluoronitrobenzene (0.375 g, 2.65 mmol) in DMF (25 mL) was passed through a Vapourtec R2+ reaction system at 0.5 mL min−1. The instrument was fitted with two 10 mL PFA coils, maintained at 100 °C and 8 bar of pressure. The resulting product stream was diluted with water (50 mL), extracted with DCM (3 × 20 mL), washed with water (3 × 40 mL) and brine (1 × 40 mL), dried (MgSO4), filtered and then concentrated under reduced pressure to give a yellow solid (0.521 g, yield 95%), mp 118–122 °C. GCMS analysis of the crude reaction material revealed greater than 95% of the desired compound.1H NMR (600 MHz, DMSO-d6) δ 8.04 (1H, d, J = 8.4 Hz), 7.00 (2H, d, J = 8.1 Hz), 3.37 (4H, s). 13C NMR (101 MHz, DMSO-d6) δ 155.1, 136.6, 125.7, 112.4, 47.3, 45.1. Mass spectrum (ESI, +ve) 179 m/z [(M + H)+, 100%] FTIR νmax (cm−1): 3334, 2948, 2838, 1580, 1480, 1309, 1244, 1100, 834, 753, 696, 665, 599.
4-Morphilinoaniline (8)
A solution of 4-(4-nitrophenyl)morpholine (13) (0.475 g, 2.28 mmol) and DMSO (3 mL) in methanol (100 mL) was pumped through a ThalesNano H-Cube® fitted with a 70 mm 10% Pd/C CatCart® at 1 mL min−1. The resulting product stream was concentrated under reduced pressure to afford a yellow oil that was then diluted with DCM (30 mL), washed with water (3 × 30 mL), saturated NaCl (30 mL), dried (Mg2SO4), filtered and then concentrated under reduced pressure to afford a tan solid (0.350 g, 87% yield). This material was identical, in all aspects, with an authentic sample and commercially available from Sigma Aldrich.17,184-(Piperazin-1-yl)aniline (9)
A solution of 1-(4-nitrophenyl)piperazine 14 (0.219 g, 1.06 mmol) in methanol (100 mL) was passed through a ThalesNano H-CubePro® using 30 mm 10% Pd/C CatCart® catalyst at 1 mL min−1 at 50 °C and 50 bar of pressure. The resulting reactant stream was concentrated under reduced pressure to afford a white solid (0.187 g, 99%) that darkened and decomposed over time on standing at room temperature. This material was deemed pure by 1H NMR spectroscopy and used in the subsequent step without further purification.1H NMR (600 MHz, CDCl3) δ 6.83–6.78 (m, 2H), 6.68–6.63 (m, 2H), 3.01 (tdd, J = 7.8, 4.0, 1.1 Hz, 8H); 13C NMR (151 MHz, CDCl3) δ 145.26, 140.25, 118.78, 116.34, 52.42, 46.46; mass spectrum (ESI, +ve) 178 m/z [(M + H)+, 100%], 279 (33), 222 (22); FTIR νmax (cm−1): 3301, 1644, 1633, 1519, 1085.
2-Amino-N-methylbenzamide (16)
To a flask containing isatoic anhydride (15) (1.86 g, 11.4 mmol), at 0 °C, methylamine (10 mL, 144.0 mmol of 40% w/v aqueous solution) at a rate of 0.25 mL min−1 using a Syrris FRX-100. The mixture was warm room temperature and stirred overnight, diluted with ethyl acetate (100 mL). The aqueous layer was extracted with ethyl acetate (3 × 100 mL), and the combined organic extracts were washed with water (200 mL), brine (2 × 200 mL), dried (MgSO4) and then concentrated under reduce pressure to afford the title compound (1.35 g, 79%), as an off-white solid.1H NMR (CDCl3, 400 MHz): δ 7.29 (1H, dd, J = 9.3, 1.5 Hz), 7.19 (1H, ddd, J = 8.6, 7.1, 1.5 Hz), 6.67 (1H, ddd, J = 8.1, 0.9 Hz), 6.65 (1H, ddd, J = 8.6, 7.2, 1.5 Hz), 6.10 (1H, bs), 5.49 (2H, bs), 2.96 (3H, d, J = 4.9 Hz); 13C NMR (CDCl3, 101 MHz): δ 170.0, 148.6, 132.2, 127.1, 117.3, 116.6, 116.3, 26.5; mass spectrum (EI+) m/z 150 (M); FTIR νmax (cm−1): 3478, 3424, 3304, 2939, 1616, 1585, 1535, 1488, 1447, 1407, 1305, 1259, 1171, 1158, 1037, 1007, 938, 859, 813, 679, 658, 561, 530, 496, 431.
2-(2,5-Dichloropyrimidin-4-ylamino)-N-methylbenzamide (10)
A stream of 2-amino-N-benzeneamide (16, 0.94 g, 6.25 mmol), 2,4,5 trichloropyrimidine (1.26 g, 6.88 mmol), iPr2NEt (1.21 g, 9.38 mmol) in isopropanol (200 mL) solution was passed through a Vapourtec R2+ reaction system at 0.25 mL min−1. The instrument was fitted with two 10 mL PFA coils, maintained at 100 °C and 4 bar of pressure. HPLC analysis of the product stream, from first-pass, revealed 71% product 10 with regard to 2-amino-N-benzeneamide (15). The volume of the resulting product stream was reduced in half, via concentration under reduced pressure, to give a white slurry. Isolation of the product 10 by vacuum filtration afforded a creamy white solid (0.54 g, 62%).1H NMR (d6-DMSO, 400 MHz): δ 12.12 (1H, s), 8.86 (1H, m), 8.51 (1H, d, J = 6 Hz), 8.46 (1H, bs), 7.80 (1H, dd, J = 7.9, 1.5 Hz), 7.61 (1H, t, J = 7.6 Hz), 7.23 (1H, t, J = 7.6 Hz), 2.82 (3H, d, J = 4.5 Hz); 13C NMR (CDCl3, 101 MHz): δ 169.2, 157.1, 156.7, 155.8, 138.6, 132.3, 128.6, 123.8, 121.8, 121.4, 115.4, 26.8; mass spectrum (ESI, +ve) 298 m/z [(M + H)+, 22%], 297 (24), 157 (100); FTIR νmax (cm−1): 3376, 2846, 1645, 1585, 1531, 1463, 1416, 1333, 1260, 1191, 1159, 1106, 1037, 925, 831, 794, 681, 657, 546, 526, 410.
2-((5-Chloro-2-((4-morpholinophenyl)amino)pyrimidin-4-yl)amino)-N-methylbenzamide (6) – CTx-0152960
To a quartz walled Smith tube was charged with 2-(2,5-dichloropyrimidin-4-ylamino)-N-methylbenzamide (10) (476 mg, 1.60 mmol), 4-morphoaniline (9) (0.158 g, 0.76 mmol) and then n-butanol (4 mL). The ensuing slurry was crimp capped and heated in the microwave (Biotage) at 150 °C for 20 min. After cooling to 18 °C, the ensuing reaction mixture was concentrated under reduced vacuum to afford a light greenish-brown solid. Subjection of this material to flash chromatography (silica, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v methanol/dichloromethane gradient elution) and concentration of the relevant fractions (Rf = 0.4 in 1
9 v/v methanol/dichloromethane gradient elution) and concentration of the relevant fractions (Rf = 0.4 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v methanol/dichloromethane) gave the title compound (0.169 g, 51%) as an off-white coloured solid (slow decomposition from 180 °C).
20 v/v methanol/dichloromethane) gave the title compound (0.169 g, 51%) as an off-white coloured solid (slow decomposition from 180 °C).
1H NMR (d6-DMSO, 400 MHz): 11.59 (1H, s), 9.22 (1H, s), 9.75–9.74 (m, 2H), 8.16 (1H, s), 7.75–7.74 (3H, m), 7.13 (1H, t, J = 6 Hz), 6.89 (2H, d, J = 8.9 Hz), 6.89 (2H, d, J = 6.9 Hz), 3.75–3.74 (4H, m), 3.06–3.04 (4H, m), 2.81 (3H, d, J = 6 Hz); 13C NMR (d6-DMSO 151 MHz) δ 170.3, 158.5, 155.6, 153.8, 147.7, 139.2, 133.0, 131.3, 127.4, 122.3, 121.9 (2C), 121.9, 121.5, 116.7 (2C), 104.9, 50.5 (2C), 45.1 (2C), 25.4; mass spectrum (ESI, +ve) 460 [(M + Na)+, 15%], 462 (8), 438 [(M), 65], 256 (54), 178 (100); FTIR νmax (cm−1) 3374, 2923, 2854, 1682, 1601, 1562, 1515, 1448, 1416, 1228, 1161, 1081, 1048, 1024, 1001, 824, 759, 593, 531.
2-((5-Chloro-2-((4-(piperazin-1-yl)phenyl)amino)pyrimidin-4-yl)amino)-N-methylbenzamide (7) – CTx-0294885
To a quartz walled Smith tube was charged with 2-(2,5-dichloropyrimidin-4-ylamino)-N-methylbenzamide (10) (0.159 g, 0.54 mmol), 4-(piperazin-1-yl)aniline (9) (0.103 g, 0.50 mmol), n-butanol (4 mL) and then HCl solution (4 drops of a 4 M dioxane solution). The ensuing slurry was crimp capped and heated in the microwave (Biotage) at 150 °C for 1 h. After cooling to 18 °C, the ensuing reaction mixture was concentrated under reduced vacuum to afford a light white solid. Subjection of this material to flash chromatography (Grace Reveleris® amino column, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v methanol/dichloromethane gradient elution) and concentration of the relevant fractions (Rf = 0.4 in 1
9 v/v methanol/dichloromethane gradient elution) and concentration of the relevant fractions (Rf = 0.4 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v methanol/dichloromethane) gave the title compound as an off-white coloured solid (0.102 mg, 47%), slow decomposition from 159 °C.
9 v/v methanol/dichloromethane) gave the title compound as an off-white coloured solid (0.102 mg, 47%), slow decomposition from 159 °C.
1H NMR (600 MHz, d4-MeOD) δ 8.71 (1H, d, J = 8.3 Hz), 8.03 (s, 1H), 7.69–7.63 (m, 1H), 7.45–7.42 (3H, dd, J = 19.3, 8.2 Hz), 7.12 (1H, t, J = 7.3 Hz), 6.97 (2H, d, J = 8.9 Hz), 3.13 (4H, d, J = 5.1 Hz), 3.04 (4H, d, J = 4.9 Hz), 2.94 (s, 3H). 13C NMR (151 MHz, d4-MeOD) δ 170.3, 158.5, 155.6, 153.8, 147.7, 139.2, 133.0, 131.3, 127.4, 122.3, 121.9 (2C), 121.9, 121.5, 116.7 (2C), 104.9, 50.5 (2C), 45.1 (2C), 25.4; mass spectrum (ESI, +ve) m/z 459 [(M + Na)+, 19%], 437 [(M), 100], 439 (33), 304 (19), 256 (38), 219 (20), 178 (100), 156 (95); FTIR νmax (cm−1): 3254, 1622, 1570, 1514, 1423, 1228, 827, 771, 750.
Acknowledgements
Financial support was received from the Australian Research Council (ARCDP 14101565), the Australian Cancer Research Foundation and the Ramaciotti Foundation.Notes and references
- P. Cohen, Nat. Rev. Drug Discovery, 2002, 1, 309–315 CrossRef CAS PubMed.
- M. Kollareddy, D. Zheleva, P. Dzubak, P. S. Brahmkshatriya, M. Lepsik and M. Hajduch, Invest. New Drugs, 2012, 30, 2411–2432 CrossRef CAS PubMed.
- N. Keen and S. Taylor, Nat. Rev. Cancer, 2004, 4, 927–936 CrossRef CAS PubMed.
- S. Davies, H. Reddy, M. Caivano and P. Cohen, Biochem. J., 2000, 351, 95–105 CrossRef CAS PubMed.
- P. Traxler, G. Bold, E. Buchdunger, G. Caravatti, P. Furet, P. Manley, T. O'Reilly, J. Wood and J. Zimmermann, Med. Res. Rev., 2001, 21, 499–512 CrossRef CAS PubMed.
- Z. O'Brien and M. Fallah Moghaddam, Expert Opin. Drug Metab. Toxicol., 2013, 9, 1597–1612 CrossRef PubMed.
- T. Yasuzawa, T. Iida, M. Yoshida, N. Hirayama, M. Takahashi, K. Shirahata and H. Sano, J. Antibiot., 1986, 1072–1078 CrossRef CAS.
- M. Bantscheff, D. Eberhard, Y. Abraham, S. Bastuck, M. Boesche, S. Hobson, T. Mathieson, J. Perrin, M. Raida, C. Rau, V. Reader, G. Sweetman, A. Bauer, T. Bouwmeester, C. Hopf, U. Kruse, G. Neubauer, N. Ramsden, J. Rick, B. Kuster and G. Drewes, Nat. Biotechnol., 2007, 25, 1035–1044 CrossRef CAS PubMed.
- J. Adachi, M. Kishida, S. Watanabe, Y. Hashimoto, K. Fukamizu and T. Tomonaga, J. Proteome Res., 2014, 13, 5461–5470 CrossRef CAS PubMed.
- F. S. Oppermann, F. Gnad, J. V. Olsen, R. Hornberger, Z. Greff, G. Keri, M. Mann and H. Daub, Mol. Cell. Proteomics, 2009, 8, 1751–1764 CAS.
- L. Margarucci, M. C. Monti, B. Fontanella, R. Riccio and A. Casapullo, Mol. BioSyst., 2011, 7, 480 RSC.
- S. Lemeer, C. Zörgiebel, B. Ruprecht, K. Kohl and B. Kuster, J. Proteome Res., 2013, 12, 1723–1731 CrossRef CAS PubMed.
- D. Brehmer, Z. Greff, K. Godl, S. Blencke, A. Kurtenbach, M. Weber, S. Müller, B. Klebl, M. Cotten, G. Kéri, J. Wissing and H. Daub, Cancer Res., 2005, 65, 379–382 CAS.
- K. Godl, J. Wissing, A. Kurtenbach, P. Habenberger, S. Blencke, H. Gutbrod, K. Salassidis, M. Stein-Gerlach, A. Missio, M. Cotten and H. Daub, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 15434–15439 CrossRef CAS PubMed.
- K. Godl, O. J. Gruss, J. Eickhoff, J. Wissing, S. Blencke, M. Weber, H. Degen, D. Brehmer, L. Orfi, Z. Horváth, G. Kéri, S. Müller, M. Cotten, A. Ullrich and H. Daub, Cancer Res., 2005, 65, 6919–6926 CrossRef CAS PubMed.
- K. Sharma, C. Weber, M. Bairlein, Z. Greff, G. Kéri, J. Cox, J. V. Olsen and H. Daub, Nat. Methods, 2009, 6, 741–744 CrossRef CAS PubMed.
- L. Zhang, I. P. Holmes, F. Hochgräfe, S. R. Walker, N. A. Ali, E. S. Humphrey, J. Wu, M. de Silva, W. J. A. Kersten, T. Connor, H. Falk, L. Allan, I. P. Street, J. D. Bentley, P. A. Pilling, B. J. Monahan, T. S. Peat and R. J. Daly, J. Proteome Res., 2013, 12, 3104–3116 CrossRef CAS PubMed.
- R. J. Daly, I. P. Holmes, I. P. Street and S. R. Walker, WO Patent WO 2013/075167, 2014, pp. 1–54.
- M. D. Hopkin, I. R. Baxendale and S. V. Ley, Org. Biomol. Chem., 2013, 11, 1822–1839 CAS.
- B. J. Deadman, M. D. Hopkin, I. R. Baxendale and S. V. Ley, Org. Biomol. Chem., 2013, 11, 1766–1800 CAS.
- I. Becher, M. M. Savitski, M. F. Savitski, C. Hopf, M. Bantscheff and G. Drewes, ACS Chem. Biol., 2013, 8, 599–607 CrossRef CAS PubMed.
- D. T. McQuade and P. H. Seeberger, J. Org. Chem., 2013, 78, 6384–6389 CrossRef CAS PubMed.
- I. R. Baxendale, L. Brocken and C. J. Mallia, Green Process. Synth., 2013, 2, 211–230 CAS.
- K. Gilmore, D. Kopetzki, J. W. Lee, Z. Horváth, D. T. McQuade, A. Seidel-Morgenstern and P. H. Seeberger, Chem. Commun., 2014, 50, 12652–12655 RSC.
- S.-H. Lau, A. Galván, R. R. Merchant, C. Battilocchio, J. A. Souto, M. B. Berry and S. V. Ley, Org. Lett., 2015, 17, 3218–3221 CrossRef CAS PubMed.
- D. Obermayer, T. N. Glasnov and C. O. Kappe, J. Org. Chem., 2011, 76, 6657–6669 CrossRef CAS PubMed.
- C. Wiles and P. Watts, Green Chem., 2012, 14, 38 RSC.
- S. V. Ley, D. E. Fitzpatrick, R. J. Ingham and R. M. Myers, Angew. Chem., Int. Ed., 2015, 54, 3449–3464 CrossRef CAS PubMed.
- R. J. Ingham, E. Riva, N. Nikbin, I. R. Baxendale and S. V. Ley, Org. Lett., 2012, 14, 3920–3923 CrossRef CAS PubMed.
- B. Biannic, J. J. Bozell and T. Elder, Green Chem., 2014, 16, 3635–3647 RSC.
- L. A. Carpino, E. Mansour, C. H. Cheng, J. R. Williams, R. MacDonald, J. Knapczyk, M. Carman and A. Lopusinski, J. Org. Chem., 1983, 48, 661–665 CrossRef CAS.
- M. A. Insalaco and D. S. Tarbell, Org. Synth., 1988, 50(9), 207–209 Search PubMed.
- T. Jong and M. Bradley, Org. Lett., 2015, 17, 422–425 CrossRef CAS PubMed.
- M. Tarleton and A. McCluskey, Tetrahedron Lett., 2011, 52, 1583–1586 CrossRef CAS.
- L. Hizartzidis, M. Tarleton, C. P. Gordon and A. McCluskey, RSC Adv., 2014, 4, 9709–9722 RSC.
- B. H. Lipshutz and K. E. McCarthy, J. Org. Chem., 1984, 49, 1218–1221 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09426g |
| This journal is © The Royal Society of Chemistry 2015 |