Abstract
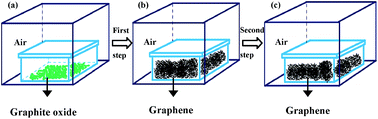
Rapid thermal exfoliation/reduction of graphite oxide is a fast and easy method among the oxidation–reduction approaches for graphene synthesis. In this research, we firstly demonstrated that graphene can be obtained with a surface area of 550 to 700 m2 g−1 and a yield of approximately 50% through one-step rapid thermal treatment in air from 450 to 550 °C, without vacuum or protective gas through a self-protection process. Then, we further demonstrated the effective two-step thermal annealing to significantly improve the C/O ratio from ca. 7.3 to 25.9 in air at the relatively low temperature of 600 °C. The smart self-protecting and enhanced-oxygen-removal mechanisms were discussed.

 Please wait while we load your content...
Please wait while we load your content...