Study on coating growth characteristics during the electrolytic oxidation of a magnesium–lithium alloy by optical emission spectroscopy analysis†
Abstract
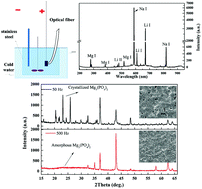
The aim of this study is to analyze the composition, structure and growth characteristics of plasma electrolytic oxidation (PEO) coatings through optical emission spectroscopy (OES). The PEO coatings were prepared on a magnesium–lithium alloy in a phosphate system at various frequencies. The composition and structure of the coatings were examined using X-ray diffraction (XRD), X-ray photo-electron spectroscopy (XPS) and scanning electron microscopy (SEM), as well as energy-dispersive X-ray (EDX). The discharge sparks of the PEO process were measured by optical emission spectroscopy. The results show that the PEO coating prepared at 50 Hz is composed of crystalline MgO and crystalline Mg3(PO4)2, and that the coating at 500 Hz is composed of crystalline MgO and amorphous Mg3(PO4)2. The coating prepared at 50 Hz has a greater degree of roughness than that prepared at 500 Hz, and the sizes of the micropores on the coating prepared at 50 Hz are considerably larger than that at 500 Hz, whereas the numbers of the micropores at various frequencies change in opposition to the pore sizes. The plasma temperature (Te) calculated with OES at 50 Hz is about 3100 K higher than that at 500 Hz. This means that more energy generated per cycle was applied to the electrode surface at 50 Hz than 500 Hz, which consequently influenced the structure and composition of the coatings. Based on the OES analysis, the growth characteristic of the PEO coatings was proposed to explain the changes of the coating roughness and the formation mechanism of crystalline or amorphous Mg3(PO4)2 at various working frequencies by the Te and the liquid-cooling effect, which was further proven by the experiments designed by changing the electrical parameters of the PEO process. This study also illustrates that the adjustment of the phase composition and structure by the electrical parameters can be well explained by OES. Besides, the corrosion resistance of the MAO coatings was evaluated by the polarization curves in 3.5 wt% NaCl solution. The corrosion resistance of the coatings is mainly determined by thickness and roughness, and the coatings prepared under 500 Hz generally present better corrosion resistance than those prepared under 50 Hz.

 Please wait while we load your content...
Please wait while we load your content...