POM-based inorganic–organic hybrid compounds: synthesis, structures, highly-connected topologies and photodegradation of organic dyes†
Xiao Li,
Liu Yang,
Chao Qin*,
Fu-Hong Liu,
Liang Zhao*,
Kui-Zhan Shao and
Zhong-Min Su*
Institute of Functional Material Chemistry, Key Laboratory of Polyoxometalate Science of Ministry of Education, Faculty of Chemistry, Department of Chemistry, Northeast Normal University, Changchun, 130024 Jilin, P. R. China. E-mail: zmsu@nenu.edu.cn; qinc703@nenu.edu.cn; zhaol352@nenu.edu.cn
First published on 17th June 2015
Abstract
Four new polyoxovanadate-based organic–inorganic hybrid materials [MnV2(bpp)2O6] (1), [Ag4V4(bpp)4O12]·2H2O (2) and [M3V6(bpp)4O18·4H2O]·2H2O [M = Ni (3), Zn (4), bpp = 1,3-bis(4-pyridyl)propane] have been synthesized under hydrothermal conditions through the self-assembly of transition metal salts, bpp ligands and ammonium metavanadate. Single-crystal X-ray diffraction analyses show that 1 possesses a three-dimensional framework, constructed from arrays of {V4O12} rings covalently linked through metal–organic units, {Mn(C13H4N2)2}. Compound 2 is an eight-connected self-catenated metal–organic framework, based on bimetallic {Ag4V4O12} clusters as nodes. Compounds 3 and 4 are isostructural, both of them exhibit an intriguing 6,10-connected network. The structure of 4, as an example, is based on single zinc atoms as six-connected nodes, as well as bimetallic {Zn2V6O18} clusters as ten-connected nodes, defining not only a new topology for three-periodic nets but also the first 6,10-connected framework using heterometallic clusters as nodes. Furthermore, the thermal stabilities and photocatalytic activities of 1–4 have been discussed in detail.
Introduction
Organic–inorganic hybrid materials based on polyoxometalates (POMs) have attracted increasing interest due to their aesthetic architectures, intriguing topologies and potential applications in a variety of fields, such as catalysis, electrochemistry, gas storage, photochemistry, and as magnetic and electromagnetic functional materials.1 It is well known that polyoxovanadates, containing many VmOnx− subunits rich with oxygen atoms that can coordinate to various metal ions, are suitable inorganic building blocks that can form polyoxovanadate-based hybrid materials.2–6 As the use of polynuclear metal clusters as building blocks is a promising strategy for the construction of highly connected coordination frameworks, polyoxovanadates are promising building blocks due to their aforementioned advantages.7 Up to now, there are a handful of highly connected networks reported based on monometallic or heterometallic clusters.8To date, a lot of N/O-donor organic ligands, including pyridine derivatives,9 pyrazine derivatives,10 imidazole derivatives,11 carboxylic acids12 and so on have been utilized to coordinate with the vanadium resulting in multiple polyoxovanadate-based hybrid materials. Compared to rigid bidentate 4,4-bipyridyl analogues, bpp ligands are flexible dipyridyl ligands with different conformations, exhibiting lots of different N-to-N distances with respect to the relative orientations of the CH2 groups and the pyridyl rings. The flexibility and conformational freedom of bpp ligands offer the possibility to meet the requirements of the coordination geometries of different ions in the assembly process and construct charming frameworks with tailored properties and functions.13 Therefore, we chose bpp as a bridging ligand to incorporate into the polyoxovanadate framework to produce unusual structures. As a result, four transition metal complex-based polyoxovanadate compounds [MnV2(bpp)2O6] (1), [Ag4V4(bpp)4O12]·2H2O (2) and [M3V6(bpp)4O18·4H2O]·2H2O [M = Ni (3), Zn (4)] were obtained, which display 4,6-, 8- and 6,10-connected topologies with different three-dimensional structures. For the 6,10-connected topology network, it is not only a new topology as a highly connected network but also the first 6,10-connected framework using heterometallic clusters as ten-connected nodes. The thermal stabilities and photocatalytic activities of 1–4 have been investigated.
Experimental section
Materials and methods
All reagents and solvents for the syntheses were purchased from commercial sources and used as received without further purification. Elemental analyses (C, H, and N) were performed on a Perkin-Elmer 240C elemental analyzer. IR spectra were recorded in the range 400–4000 cm−1 on an Alpha Centauri FT/IR spectrophotometer using KBr pellets. PXRD patterns were collected using a Siemens D5005 diffractometer with Cu-Kα (λ = 1.5418 Å) radiation in the range of 3–50° at 293 K. Thermogravimetric analyses (TGA) were conducted on a Perkin-Elmer TG-7 analyzer heated from room temperature to 800 °C under a nitrogen atmosphere at a rate of 10 °C min−1. The UV/Vis absorption spectra were recorded on a Shimadzu UV-2550 spectrophotometer in the wavelength range of 200–800 nm. Inductively coupled plasma (ICP) analyses were conducted on a Leeman Laboratories Prodigy inductively coupled plasma-optical atomic emission spectrometry (ICP-AES) system.Syntheses of compounds 1–4
X-ray crystallography
Single-crystal X-ray diffraction data for 1–4 were collected on a Bruker SMART APEX II CCD diffractometer equipped with a graphite monochromator using Mo-Kα radiation (λ = 0.71073 Å) by using the Φ/ω scan technique at room temperature; there was no evidence of crystal decay during data collection. A multiscan technique was used to perform adsorption corrections. The crystal structures of 1–4 were solved using direct methods and refined using the full-matrix least-squares method on F2 with anisotropic thermal parameters for all non-hydrogen atoms using the SHELXL-97 program.14 Relevant crystal data and structure refinements for 1–4 are listed in Table 1. Selected bond lengths (Å) and angles (°) for 1–4 are given in Table S1.†| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| a R1 = ∑‖Fo| − |Fc‖/∑|Fo|.b wR2 = |∑w(|Fo|2 − |Fc|2)|/∑|w(Fo2)2|1/2. | ||||
| Chemical formula | C26H28Mn4O6V2 | C52H56Ag4N8O15V4 | C52H62N8Ni3O24V6 | C52H56N8O24V6Zn3 |
| Formula weight | 649.34 | 1668.29 | 1664.87 | 1678.80 |
| Crystal system | Tetragonal | Monoclinic | Monoclinic | Monoclinic |
| Space group | I4(1)/a | P2/n | P2(1)/n | P2(1)/n |
| a (Å) | 21.5654(11) | 12.382(3) | 13.081(3) | 13.1897(16) |
| b (Å) | 21.5654(11) | 9.697(2) | 16.379(4) | 16.438(2) |
| c (Å) | 13.0149(8) | 25.365(6) | 15.407(4) | 15.4725(19) |
| α (°) | 90 | 90 | 90 | 90 |
| β (°) | 90 | 92.003(4) | 102.368(5) | 102.345(2) |
| γ (°) | 90 | 90 | 90 | 90 |
| V (Å3) | 6052.8(6) | 3043.5(13) | 3224.4(14) | 3277.0(7) |
| Temperature (K) | 293(2) | 293(2) | 293(2) | 293(2) |
| Z | 8 | 2 | 2 | 2 |
| Dcalcd (g cm−3) | 1.425 | 1.820 | 1.715 | 1.701 |
| GOF on F2 | 1.061 | 0.937 | 1.013 | 1.020 |
| R1 [I > 2σ(I)]a | 0.0393 | 0.0665 | 0.0376 | 0.0557 |
| wR2 [I > 2σ(I)]b | 0.1089 | 0.1537 | 0.0844 | 0.1431 |
| R1a (all data) | 0.0551 | 0.1280 | 0.0632 | 0.0946 |
| wR2b (all data) | 0.1173 | 0.1890 | 0.0968 | 0.1667 |
| Rint | 0.0502 | 0.0963 | 0.0460 | 0.0728 |
Results and discussion
Crystal structure of [MnV2(bpp)2O6] (1)
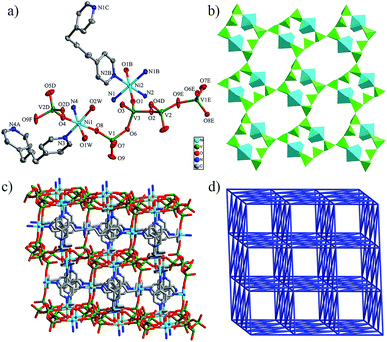
The single-crystal X-ray diffraction analysis of 1 reveals that it crystallizes in the tetragonal group I4(1)/a. As shown in Fig. 1a, the asymmetric unit of 1 consists of a half Mn(II) ion, one bpp ligand and one V cation of a {V4O12} ring. In the {V4O12} ring, all four vanadium atoms show a VO4 tetrahedron coordination configuration, and each vanadium centre is coordinated with three bridging oxygen atoms (Ob) and one terminal oxygen atom (Ot). The V–Ot distance of 1.615(3) Å is slightly shorter than the V–Ob distances of 1.772(3) and 1.651(2) Å. The Mn1 ion is six-coordinated by four nitrogen atoms from four bpp ligands and two oxygen atoms from two adjacent {V4O12} rings. The Mn–N bond lengths are 2.189(3) and 2.224(3) Å, and the Mn–O bond length is 1.995(2) Å, which are summarized in Table S1.† Furthermore, all of the bpp ligands adopt a trans–gauche conformation.15In 1, the adjacent {V4O12} rings are linked to Mn ions to form neutral bimetallic oxide layers {Mn2V4O12} (Fig. 1b) with channels occupied by bpp ligands which cross-link the {Mn2V4O12} motifs to generate a three dimensional framework, as shown in Fig. 1c. In order to simplify the 3D framework of 1, the Mn1 ion is regarded as a six-connected node and the {V4O12} rings can be considered as four-connected nodes. Thus, 1 exhibits a binodal (4,6)-connected 3D net with a (44·55·66)2(54·62) topology when analysed by TOPOS software (Fig. 1d).
Crystal structure of [Ag4V4(bpp)4O12]·2H2O (2)
2 contains neutral bimetallic {Ag4V4O12} clusters as nodes, according to the single-crystal X-ray analysis, which consist of a central {V4O12}4− cluster decorated with four Ag units, as shown in the insert of Fig. 2a. There are two crystallographically independent vanadium atoms in 2, which show a similar distorted tetrahedron to 1.814(7) Å (Fig. 2a). Tetrahedral and planar-trigonal coordination geometries of Ag ions are presented in Fig. 2a. The Ag1 ion is coordinated with two nitrogen atoms from two bpp ligands and two terminal oxygen atoms from one {V4O12}4− anion, constructing a tetrahedral geometry. The Ag2 ion is coordinated with two nitrogen atoms from two bpp ligands and one terminal oxygen atom from one {V4O12}4− anion to form a planar-trigonal geometry. The average Ag–O distance [2.636(3)Å] and Ag–N distance [2.222(8) Å] in the tetrahedron is longer than the corresponding values [2.371(8) Å and 2.145(9) Å] in the trigonal geometry. All of the bpp ligands exhibit a trans–trans conformation with the present N–N distances ranging from 9.718(0) to 10.191(4) Å. Fig. 2b shows that the Ag ions in adjacent {Ag4V4O12} clusters have Ag⋯Ag interactions with short silver–silver contacts of 3.0767(19) and 3.2069(18) Å, which are shorter than the sum of the van der Waals radii of the two silver atoms (3.440(0) Å).16 Every {Ag4V4O12} cluster is further connected to its eight nearest neighbours through eight bpp ligands, thus resulting in the three-dimensional framework illustrated in Fig. 2c.From the viewpoint of the topology, each bimetallic {Ag4V4O12} cluster is surrounded by eight bridging bpp ligands, further connecting eight {Ag4V4O12} clusters with distances of 12.238(2) to 22.853(2) Å, defining an eight-connected node. In this bridging of parallel layers, the catenated four-membered shortest rings are observed at the intersection of the crossing of two-dimensional layers, therefore resulting in a single eight-connected self-catenated network (Fig. 2d). The total Schlafli symbol of this net is (424·64). It is worth noting that the first self-catenated framework using heterometallic clusters as nodes was [Cu4(bpp)4V4O12]·3H2O [bpp = 1,3-bis(4-pyridyl)propane] reported in 2007.17
Crystal structure of [Ni3V6(bpp)4O18·4H2O]·2H2O (3)
3 and 4 are isostructural, so the structural description of 3 is given as a representative example. The single-crystal X-ray diffraction analysis reveals that 3 crystallized in the monoclinic space group P2(1)/n. As shown in Fig. 3a, the asymmetric unit of 3 consists of one and a half Ni(II) ions, two bpp ligands and three vanadium atoms. All of the vanadium atoms exhibit a {VO4} tetrahedron coordination configuration, and each vanadium centre is coordinated with three bridging oxygen atoms (Ob) and one terminal oxygen atom (Ot). The V–Ob distances range from 1.650(2) to 1.809(2) Å and are slightly longer than the values of V–Ot distances of 1.614(2) to 1.650(2) Å. Ni ions are six-coordinated showing an octahedral geometry coordination but different coordination environments. The Ni1 ion is coordinated with two nitrogen atoms from two bpp ligands and four oxygen atoms from two vanadium tetrahedra and two water molecules, while the Ni2 ion is coordinated with four nitrogen atoms from four bpp ligands and two oxygen atoms from two vanadium tetrahedra. The Ni–N bond lengths are in the range of 2.077(2)–2.158(2) Å and the Ni–O distances vary from 2.042(2) to 2.113(3) Å (Table S1†). All of the bpp ligands show trans–gauche conformations with the N–N distances in the range 8.791(9) to 9.267(3) Å.In 3, two Ni1 ions and six vanadium atoms are bridged by eight bridging oxygen atoms to generate a bimetallic {Ni2V6O20}6− clusters. Each {Ni2V6O20}6− cluster links four neighbours by a V2–O9–V1 bridge to form 2D inorganic layers, given in Fig. 3b. The 2D inorganic layers are further connected by the Ni2 ions through bridging oxygen atoms into a 3D framework (Fig. 3c). From a topological point of view, each {Ni2V6O20}6− cluster is surrounded by four bpp ligands, four neighbouring {Ni2V6O20}6− clusters and two Ni1 ions, which defines a bimetallic ten-connected node. Likewise, a Ni2 ion is surrounded by two {Ni2V6O20}6− clusters and four bpp ligands, thus defining a six-connected node. Therefore, 3 presents a binodal (6,10)-connected 3D net with a point symbol of (413·62)(436·69) in which each {Ni2V6O20}6− cluster is linked to ten nearest-neighbours (eight {Ni2V6O20}6− clusters and two Ni2 ions) and each Ni2 ion is linked to six nearest-neighbours (two {Ni2V6O20}6− clusters and four Ni2 ions) with distances of 6.594–13.882 Å (centre to centre), as shown in Fig. 3d.
To the best of our knowledge, only one (6,10)-connected structure has been reported up to now.18 The example is [Zn7(trz)6(pt)4(H2O)2] (Htrz = 1,2,4-triazole, H2pt = isophthalic acid) based on binuclear zinc clusters as six-connected nodes and pentanuclear zinc clusters as ten-connected nodes, which is the first binodal (6,10)-connected metal–organic network with the topology of (34·48·53)(38·420·512·65).
Thermogravimetric analyses
To study the thermal stabilities of 1–4, the thermogravimetric analyses were carried out under a N2 atmosphere from room temperature to 1000 °C with a heating rate of 10 °C min−1. 1 shows a single weight loss step (Fig. S2†). The loss step (180–240 °C for 1) corresponds to the decomposition of the bpp ligands and the {V4O12} clusters. 2–4 exhibit three separate weight loss steps (Fig. S2†). The first weight loss (120–200 °C for 2–4) corresponds to the release of interstitial water molecules (found 3.21% for 2, 6.54% for 3, 6.47% for 4; calcd 4.11% for 2, 5.35% for 3, 6.04% for 4). The second and third weight losses (300–800 °C for 2, 270–800 °C for 3 and 4) are attributed to the decomposition of the bpp ligands and the {Ag4V4O12} clusters for 2, and the {M2V6O20} cluster for 3 and 4 [M = Ni (3), Zn (4)]. The remaining products should be MO (M = Mn, Ni, Zn), M2O (M = Ag) and V2O5 (found 38.93% for 1, 49.64% for 2, 46.25% for 3, 47.13% for 4; calcd 38.46% for 1, 48.27% for 2, 5.82% for 3, 46.55% for 4).Photocatalytic activity
It is well known that a wide range of polyoxovanadate-based compounds possess photocatalytic activities.19 Herein, methylene blue (MB), a typical, difficult-to-degrade dye contaminant molecule present in waste water, was selected to evaluate the photocatalytic effectiveness of 1–4. It is worth noting that 1–4 cannot be dissolved in aqueous solution, thus they are used as heterogeneous catalysts. The photocatalytic reactions were performed as follows: 50 mg of the catalyst (1–4) was dispersed in a 300 mL aqueous solution of MB (10 mg L−1) under stirring in the dark for 30 min in order to rule out the effect of its absorption to the particle surfaces. Then the solution was exposed to UV light (125 W) and kept continuously stirring. Samples of 5 mL were taken out every 15 min for UV measurement. The photodegradation process of MB without any photocatalyst was also performed for comparison.Fig. 4 and 5 show the photocatalytic results of 1–4 in the MB solution. We can see that the absorption peaks of aqueous MB in the presence of 1–4 dramatically decreased within 90 min under UV irradiation from Fig. 4. It is obvious that 1–4 all show good photocatalytic activity for the degradation of MB. Furthermore, based on the plots of Ct/C0 versus reaction time (Fig. 5), it can be observed that the photocatalytic activities obviously increase from 25.8% to 79.9, 85.1, 77.4, 69.5% for 1–4, respectively, after 90 min of irradiation. The PXRD patterns of 1–4 simulated from single-crystal X-ray data, as-synthesized and after the photocatalytic reaction, are shown in Fig. S2.† The PXRD results reveal that 1–4 remain unchanged after the photocatalytic reaction, indicating that they are stable photocatalysts. Moreover, because 2 exhibits the highest catalytic activity in the degradation of MB under UV light, it was chosen as a representative catalyst to investigate catalyst lifetime. In our initial experiment, the catalytic activity of 2 was maintained when it was recycled five times (Fig. S5†). According to the related literature,20 the photocatalytic mechanisms may be deduced as follows: during the photocatalytic process, there is an electron transfer from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) which is produced from the POM, induced by UV light. The HOMO strongly needs an electron in order to return to its stable state. Therefore, one electron is abstracted from a H2O molecule, which is oxygenated into the ˙OH active species. Then the ˙OH radicals could cleave MB effectively to complete the photocatalytic process.
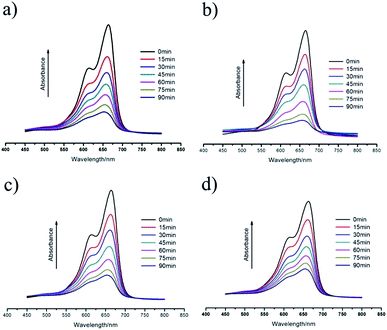 | ||
| Fig. 4 (a–d) UV/Vis absorption spectra of aqueous MB during the decomposition reaction under UV light irradiation in the presence of 1–4, respectively. | ||
 | ||
| Fig. 5 Plot of Ct/C0 of MB versus irradiation time under UV light in the presence of 1–4 (1 (▲); 2 (●); 3 (★), 4 (■); experiment in the absence of catalyst (▶)). | ||
Conclusions
Four new polyoxovanadate-based hybrid materials constructed from {V4O12} clusters, {Ag4V4O12} clusters and {M2V6O20} clusters have been prepared under hydrothermal conditions, which all exhibit 3D frameworks with different topologies. 3 and 4 show a new complicated 6,10-connected topology. 2 shows good photocatalytic activity and selectivity for the degradation of MB. This work provides useful information for designing and constructing intriguing architectures of high dimensional and highly connected multifunctional polyoxovanadate-based hybrid materials.Acknowledgements
This work was financially supported by the NSFC of China (no. 21471027, 21171033, 21131001, 21222105), the National Key Basic Research Program of China (no. 2013CB834802), and the Changbai mountain scholars of Jilin Province.Notes and references
- (a) D. E. Katsoulis, Chem. Rev., 1998, 98, 359 CrossRef CAS PubMed; (b) L. Carlucci, G. Ciani and D. M. Proserpio, Coord. Chem. Rev., 2003, 246, 247 CrossRef CAS; (c) L. Cronin, Chem. Soc. Rev., 2007, 36, 105 RSC; (d) J. Zhang, Y. F. Song, L. Cronin and T. B. Liu, J. Am. Chem. Soc., 2008, 130, 14408 CrossRef CAS PubMed; (e) D. L. Long, E. Burkholder and L. Cronin, Chem. Soc. Rev., 2007, 36, 105 RSC; (f) S. T. Zheng and G. Y. Yang, Chem. Soc. Rev., 2012, 41, 7623 RSC; (g) D. Y. Du, L. K. Yan, Z. M. Su, S. L. Li, Y. Q. Lan and E. B. Wang, Coord. Chem. Rev., 2013, 257, 702 CrossRef CAS PubMed; (h) A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009 CrossRef CAS PubMed.
- (a) V. Soghomonian, Q. Chen, R. C. Haushalter and J. Zubieta, Angew. Chem., Int. Ed., 1995, 34, 223 CrossRef CAS PubMed; (b) C. L. Chen, A. M. Goforth, M. D. Smith, C. Y. Su and H. C. Z. Loye, Angew. Chem., Int. Ed., 2005, 44, 6673 CrossRef CAS PubMed; (c) L. Luo and P. A. Maggard, Cryst. Growth Des., 2013, 13, 5282 CrossRef CAS.
- (a) R. F. de Luis, J. Orive, E. S. Larrea, M. K. Urtiaga and M. I. Arriortua, Cryst. Growth Des., 2014, 14, 658 CrossRef; (b) G. J. Cao, S. T. Zheng, W. H. Fang and G. Y. Yang, Inorg. Chem. Commun., 2010, 13, 834 CrossRef CAS PubMed; (c) R. F. Luis, M. K. Urtiaga, J. L. Mesa, J. O. G. Segura, T. Rojo and M. I. Arriortua, CrystEngComm, 2011, 13, 6488 RSC.
- (a) G. C. Ou, L. Jiang, X. L. Feng and T. B. Lu, Dalton Trans., 2009, 71 RSC; (b) Y. Q. Lan, S. L. Li, Z. M. Su, K. Z. Shao, J. F. Ma, X. L. Wang and E. B. Wang, Chem. Commun., 2008, 44, 58 RSC; (c) J. Thomas, M. Agarwal, A. Ramanan, N. Chernova and M. S. Whittingham, CrystEngComm, 2009, 11, 625 RSC.
- (a) Y. L. Teng, B. X. Dong, J. Peng, S. Y. Zhang, L. Chen, L. Song and J. Ge, CrystEngComm, 2013, 15, 2783 RSC; (b) Z. B. Zhang, Y. Xu, L. Zheng, D. R. Zhu and Y. Song, CrystEngComm, 2011, 13, 2191 RSC; (c) Y. Hu, F. Luo and F. F. Dong, Chem. Commun., 2011, 47, 761 RSC.
- X. L. Wang, C. H. Gong, J. W. Zhang, L. L. Hou, J. Luan and G. C. Liu, CrystEngComm, 2014, 16, 7745 RSC.
- (a) S. B. Li, W. Zhu, H. Y. Ma, H. J. Pang, H. Liu and T. T. Yu, RSC Adv., 2013, 3, 9770 RSC; (b) X. L. Wang, C. Qin, E. B. Wang, Z. M. Su, Y. G. Li and L. Xu, Angew. Chem., Int. Ed., 2006, 45, 7411 CrossRef CAS PubMed; (c) X. L. Wang, C. Qin, E. B. Wang and Z. M. Su, Chem.–Eur. J., 2006, 12, 2680 CrossRef CAS PubMed; (d) X. L. Wang, C. Qin, E. B. Wang, Z. M. Su, L. Xu and S. R. Batten, Chem. Commun., 2005, 4789 RSC; (e) J. Yang, B. Li, J. F. Ma, Y. Y. Liu and J. P. Zhang, Chem. Commun., 2010, 46, 8383 RSC; (f) Q. G. Zhai, J. P. Niu, S. N. Li, Y. C. Jiang, M. C. Hu and S. R. Batten, CrystEngComm, 2011, 13, 4508 RSC.
- (a) S. L. Cai, S. R. Zheng, Z. Z. Wen, J. Fan and W. G. Zhang, Cryst. Growth Des., 2012, 12, 5737 CrossRef CAS; (b) K. H. He, W. C. Song, Y. W. Li, Y. Q. Chen and X. H. Bu, Cryst. Growth Des., 2012, 12, 1064 CrossRef CAS; (c) X. L. Wang, C. Qin, Y. Q. Lan, K. Z. Shao, Z. M. Su and E. B. Wang, Chem. Commun., 2009, 410 RSC.
- (a) R. F. Luis, J. L. Mesa, M. K. Urtiaga, T. Rojo and M. I. Arriortua, Eur. J. Inorg. Chem., 2009, 32, 4786 CrossRef PubMed; (b) R. F. Luis, M. K. Urtiaga, J. L. Mesa, A. T. Aguayo, T. Rojo and M. I. Arriortua, CrystEngComm, 2010, 12, 1880 RSC.
- (a) E. S. Larrea, J. L. Mesa, J. L. Pizarro, M. I. Arriortua and T. Rojo, J. Solid State Chem., 2007, 180, 1149 CrossRef CAS PubMed; (b) J. Thomas, M. Agarwal, A. Ramanan, N. Chernov and M. S. Whittingham, CrystEngComm, 2009, 11, 625 RSC.
- (a) Y. Q. Lan, S. L. Li, X. L. Wang, K. Z. Shao, D. Y. Du, Z. M. Su and E. B. Wang, Chem.–Eur. J., 2008, 14, 9999 CrossRef CAS PubMed; (b) X. L. Wang, B. K. Chen, G. C. Liu, H. Y. Lin, H. L. Hu and Y. Q. Chen, J. Inorg. Organomet. Polym., 2009, 19, 176 CrossRef CAS; (c) J. X. Meng, Y. Lu, Y. G. Li, H. Fu and E. B. Wang, Cryst. Growth Des., 2009, 9, 4116 CrossRef CAS.
- (a) T. S.-C. Law, H. H.-Y. Sung and I. D. Williams, Inorg. Chem. Commun., 2000, 3, 420 CrossRef CAS; (b) C. L. Pan, J. Q. Xu, K. X. Wang, X. B. Cui, L. Ye, Z. L. Lu, D. Q. Chu and T. G. Wang, Inorg. Chem. Commun., 2003, 6, 370 CrossRef CAS.
- (a) S. Wang, H. Xing, Y. Z. Li, J. F. Bai, Y. Pan, M. Scheer and X. Z. You, Eur. J. Inorg. Chem., 2006, 3041 CrossRef CAS PubMed; (b) W. H. Zhang, J. P. Lang, Y. Zhang and B. F. Abrahams, Cryst. Growth Des., 2008, 8, 399 CrossRef CAS; (c) J. Qian, H. Yoshikawa, J. F. Zhang, H. J. Zhao, K. Awaga and C. Zhang, Cryst. Growth Des., 2009, 9, 5351 CrossRef CAS; (d) P. Lin, W. Clegg, R. W. Harrington, R. A. Henderson, A. J. Fletcher, J. Bell and K. M. Thomas, Inorg. Chem., 2006, 45, 4284 CrossRef CAS PubMed; (e) X. L. Wang, Y. F. Bi, B. K. Chen, H. Y. Lin and G. C. Liu, Inorg. Chem., 2008, 47, 2442 CrossRef CAS PubMed.
- (a) G. M. Sheldrick, SHELXS97, A Program for the Solution of Crystal Structures from X-ray Data, University of Göttingen, Germany, 1997 Search PubMed; (b) G. M. Sheldrick, SHELXL97, A Program for the Refinement of Crystal Structures from X-ray Data, University of Göttingen, Göttingen, Germany, 1997 Search PubMed; (c) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- (a) L. Carlucci, G. Ciani, D. M. Proserpiob and S. Rizzato, CrystEngComm, 2002, 4, 121 RSC; (b) M. V. Marinho, M. I. Yoshida, K. J. Guedes, K. Krambrock, A. J. Bortoluzzi, M. Horner, F. C. Machado and W. M. Teles, Inorg. Chem., 2004, 43, 1539 CrossRef CAS PubMed; (c) L. Pan, E. B. Woodlock, X. Wang, K. C. Lam and A. L. Rheingold, Chem. Commun., 2001, 1762 RSC; (d) Y. M. Dai, E. Tang, J. F. Huang, Y. G. Yao and X. D. Huang, J. Mol. Struct., 2009, 918, 183 CrossRef CAS PubMed.
- Y. F. Qi, E. B. Wang, J. Li and Y. G. Li, J. Solid State Chem., 2009, 182, 2640 CrossRef CAS PubMed.
- X. S. Qu, L. Xu, G. G. Gao, F. Y. Li and Y. Y. Yang, Inorg. Chem., 2007, 46, 4775 CrossRef CAS PubMed.
- J. Qin, C. Qin, C. X. Wang, H. Li, L. Cui, T. T. Li and X. L. Wang, CrystEngComm, 2010, 12, 4071 RSC.
- (a) H. S. Lin and P. A. Maggard, Inorg. Chem., 2008, 47, 8051 Search PubMed; (b) Y. Hu, F. Luo and F. F. Dong, Chem. Commun., 2011, 47, 763 Search PubMed; (c) Q. Wu, X. L. Hao, X. J. Feng, Y. H. Wang, Y. G. Li, E. B. Wang, X. Q. Zhu and X. H. Pan, Inorg. Chem. Commun., 2012, 22, 139 CrossRef PubMed; (d) C. C. Chen, W. Zhao, P. X. Lei, J. C. Zhao and N. Serpone, Chem.–Eur. J., 2004, 10, 1956 CrossRef CAS PubMed; (e) X. L. Hao, Y. Y. Ma, Y. H. Wang, L. Y. Xu, F. C. Liu, M. M. Zhang and Y. G. Li, Chem.–Asian. J., 2014, 9, 819 CrossRef CAS PubMed.
- (a) Y. Q. Chen, G. R. Li, Y. K. Qu, Y. H. Zhang, K. H. He, Q. Gao and X. H. Bu, Cryst. Growth Des., 2013, 13, 901 CrossRef CAS; (b) J. W. Sun, M. T. Li, J. Q. Sha, P. F. Yan, C. Wang, S. X. Li and Y. Pan, CrystEngComm, 2013, 15, 10584 RSC; (c) X. L. Wang, J. Luan, F. F. Sui, H. Y. Lin, G. C. Liu and C. Xu, Cryst. Growth Des., 2013, 13, 3561 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The materials, synthesis methods, BVS, XRD, XPS, TGA, ESI-MS, UV-Vis diffuse reflectance spectra. CCDC 1063206–1063209. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra09339b |
| This journal is © The Royal Society of Chemistry 2015 |