Molecular insight into the dynamical adsorption behavior of nanoscale water droplets on a heterogeneous surface†
Abstract
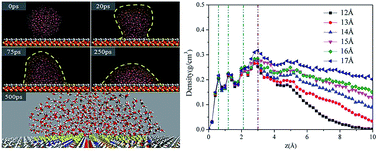
Owing to the outstanding and extraordinary wetting properties, heterogeneous surfaces have extensive and intriguing applications, such as microfluidic systems, inkjet printing and protein hydration. In this work, a heterogeneous surface is constructed by adding one hydrophilic patch at the center of a hydrophobic surface, and the dynamical adsorption process of nanoscale water droplets with different sizes on the heterogeneous surface is investigated adopting molecular dynamics simulations. The detailed adsorption processes and microscopic parameters including contact angle, density distribution profile, interaction energy, etc. are analyzed. The results present that as the droplet size increases, the water droplet would experience spreading, restricting, vibrating and slipping on the heterogeneous surface. In the process of spreading, all the water molecules spread on the hydrophilic patch under the hydrogen-bond interactions. As the droplet size increases, the pre-adsorbed water molecules play the role of an “anchor”, promoting the adsorption of the rest of the water molecules and restricting the water droplets within the hydrophilic patch. By increasing the droplet size further, some water molecules would get rid of the restriction from the pre-adsorbed water molecules and vibrate at the boundary of the hydrophilic patch. Finally, during the process of slipping, the wetting behaviors of the water droplet are dominated by the hydrophobic surface. The wetting behaviors discussed here are helpful for comprehensive exploration of the wetting behaviors of various solutions on the heterogeneous surface at the molecular level, and the present research might trigger further studies on the applications of the homogeneous surfaces.

 Please wait while we load your content...
Please wait while we load your content...