Poly(2-aminothiazole) as a unique precursor for nitrogen and sulfur co-doped porous carbon: immobilization of very small gold nanoparticles and its catalytic application†
Yasamin Bide,
Mohammad Reza Nabid* and
Fateme Dastar
Faculty of Chemistry, Department of Polymer, Shahid Beheshti University, G.C., P.O. Box 1983969411, Tehran, Iran. E-mail: m-nabid@sbu.ac.ir; Fax: +98 21 22431661; Tel: +98 21 29903102
First published on 13th July 2015
Abstract
In this contribution, we report two important achievements; first the synthesis of poly(2-aminothiaozle) (P2AT) with a plate structure containing nanoparticles by a rapidly initiated strategy, and second, the one-step synthesis of nitrogen and sulfur co-doped porous carbon materials with unique morphology using P2AT as the source of both N and S, through a simple hydrothermal carbonization reaction. The obtained functional carbon may serve to develop new carbon materials for various applications, especially catalysis, because of the excellent properties, such as straightforward synthesis, defined morphology, high porosity, and heteroatom doping. As one of the most important applications, we used the porous carbon materials as a support for immobilizing gold nanoparticles (AuNPs). Benefiting from the synergistic effects of N and S co-doping, very small AuNPs, and unique structural features, the as-obtained material was demonstrated to be a superior catalyst for reduction of nitrobenzenes.
1. Introduction
The extensive availability of carbon-based materials and also their various physicochemical properties such as electrical conductivity, chemical and thermal stability, as well as their ability to adopt different morphologies, make them suitable for wide applications such as energy storage,1,2 catalysis,3–5 sorption, and chromatography. The high surface area and porosity are desirable properties for most of these applications and especially for catalysis.6,7Therefore, much effort has been paid by researchers to design new types of functional carbons through special precursors, surface decoration and meso-/nanopore engineering due to the their properties.8 The modification of carbon-based compounds through heteroatom doping may become the “Next Big Thing” in materials science.9 Because of the large electronegativity of nitrogen (3.04) in comparison with C (2.55),10 it is the most extensively adopted element among all doping elements. Improving the electric conductivity and increasing chemical stability are just two advantages of nitrogen doping.11,12 Multiple doping as a useful synthetic approach for novel carbon materials modifies the carbon properties one step further compared to a one-type-only heteroatom. The co-doping of nitrogen and the other elements such as P, B (with lower electronegativity than C), and S (similar electronegativity with C)13,14 has been investigated. Among them, sulfur doping in carbon materials is still quite rare and further research is expected due to its unique properties.15 According to the density functional theory (DFT) calculations conducted by Qiao et al., a synergistic interaction of the N- and S-dopants existed, probably because of easier polarizability.16
Several methods such as in situ doping and post-treatment have been suggested to introduce heteroatoms into the carbon matrix.17,18 Besides the more complicated process, the main disadvantage of post treatment strategy is the inhomogeneous distribution of heteroatoms and consequently ineffective improvement of the bulk properties. In contrast, the in situ doping method includes the direct carbonization of heteroatom-containing compounds such as ionic liquids or polymers, which allows us to obtain a homogeneous distribution of heteroatoms in the carbon matrix.19–22
2-Aminothiazole (2-AT), as a nitrogen and sulfur-containing aromatic heterocyclic compound, is a biologically active material with several useful properties, such as anti-corrosion,23 antimicrobial,24 and antitumor.25 So far, only a few reports exist on the polymerization of 2-AT,26,27 which represents an emerging field in polymer research due to the special structure of this polymer for modern applications. To the best of our knowledge, there is no report on employing a rapid mixing reaction route for synthesis of P2AT wherein sufficient mixing can be easily attained by stirring or shaking before the polymerization begins.28–30 In this work, P2AT with a special plate structure containing nanoparticles was synthesized using rapid mixing polymerization, and then it was chosen as a single source precursor for the synthesis of N and S co-doped porous carbon materials ((N, S)-PCM) through a hydrothermal carbonization route. Structurally, a mesoporous and macroporous structure in the (N, S)-PCM is achieved. Chemically, the (N, S)-PCM displays a high amount of heteroatom-doping, with N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C and S
C and S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios of up to 1
C ratios of up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 (sulfur and nitrogen contents up to 4.45 and 14.2 at%), respectively. We expected that, due to the high affinity of gold to S and N, the dual doped carbon support can enhance Au loading with uniform dispersion. Thanks to gold-based catalysts, numerous organic reactions have been reachable under simple conditions with both high yields and chemoselectivity.31 It should be notable that the catalytic activity of gold nanoparticles decreases considerably as the particle size grows beyond 10 nm.32
13 (sulfur and nitrogen contents up to 4.45 and 14.2 at%), respectively. We expected that, due to the high affinity of gold to S and N, the dual doped carbon support can enhance Au loading with uniform dispersion. Thanks to gold-based catalysts, numerous organic reactions have been reachable under simple conditions with both high yields and chemoselectivity.31 It should be notable that the catalytic activity of gold nanoparticles decreases considerably as the particle size grows beyond 10 nm.32
Continuing our interest in the development of various nano-catalysts for organic transformations,21,22,33–37 herein we employed gold nanoparticles immobilized on a N and S co-doped porous carbon structure (AuNPs@(N, S)-PCM) for catalytic reduction of nitrobenzenes. The presence of hydrogen gas as a highly flammable substance can pose safety concerns.38 So, the as prepared catalyst was employed for reduction of nitrobenzenes with NaBH4 as the hydrogen source because of its non-flammability, easily hydrolyzable nature and controlled H2 production rate.39
This (N, S)-PCM not only presents a very promising material for catalytic applications, but can also be employed in other fields such as lithium–air batteries, extraction, sensors, and drug delivery.
2. Experimental
2.1. Materials
Tetrachloroauric(III) acid trihydrate 99.5%, sodium hypochlorite solution, sodium borohydride, and 2-aminothiazole were purchased from Merck Chem. Co. All other chemicals were purchased from Aldrich or Merck companies and used as received without any further purification.2.2. Instruments and characterization
Fourier transform infrared (FT-IR) spectra were recorded on a Bomem MB-Series FT-IR spectrophotometer. Transmission electron microscopy (TEM) was performed by LEO 912AB electron microscope. Ultrasonic bath (EUROSONIC® 4D ultrasound cleaner with a frequency of 50 kHz and an output power of 350 W) was used to disperse the materials in the solvents. Thermogravimetric analysis (TGA) was carried out using a STA 1500 instrument at a heating rate of 10 °C min−1 in air. The elemental analysis (CHN) was done by a conventional combustion method based on burning off the sample, and detecting the gases by a thermo conductivity detector (TCD). X-ray powder diffraction (XRD) data were collected on an XD-3A diffractometer using Cu Kα radiation. X-ray photoelectron spectroscopy (XPS) was performed using a VG multilab 2000 spectrometer (ThermoVG scientific) in an ultrahigh vacuum. Ultraviolet-Visible (UV-Vis) spectra were obtained using a Shimadzu UV-2100 spectrophotometer. Scanning electron microscope (SEM) was performed on a Zeiss Supra 55 VP SEM instrument. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) equation using a PHS-1020(PHSCHINA) device. Pore size distribution was determined by the Barrett–Joyner–Halenda (BJH) method. The samples were degassed at 150 °C for 20 h before measurements. AA-680 Shimadzu (Kyoto, Japan) flame atomic absorption spectrometer (AAS) with a deuterium background corrector was used for determination of the metal. The conversions were obtained using a Varian 3900 GC.2.3. Synthesis of poly(2-aminothiazole)
The special morphology of P2AT has been synthesized under rapidly initiated conditions. In a typical synthesis, 25 mL aqueous solution of 2-aminothiazole (0.13 M) was mixed with 25 mL aqueous solution of acetic acid (0.13 M) under ultrasonic stirring for 2 min. Then 25 mL aqueous solution of sodium hypochlorite (0.065 M) as an oxidant was added into the above mixture quickly and with immediate stirring or shaking to ensure sufficient mixing before polymerization begins. The solutions were cooled in a refrigerator at 10 °C for 10 min before the reactants were mixed. The polymerization reaction was carried out for 12 h at the specified temperature without any disturbance. The reaction mixture was neutralized with a 0.01 M KOH solution. The resulting solid product was isolated by centrifugation and washed with MeCN (2 × 30 mL) and hot water (2 × 30 mL) to separate out the unreacted monomer and mineral salts, respectively. Finally, the product was dried under vacuum at room temperature for 24 h (Scheme 1).2.4. Synthesis of N and S co-doped porous carbon structure derived from P2AT
The N and S co-doped porous carbon structure was synthesized with a combined hydrothermal and freeze-drying process. Typically, P2AT was firstly dispersed in water with ultrasonication reaching a concentration up to 3 mg mL−1. A 5 mL aqueous dispersion of P2AT (3 mg mL−1) was sonicated for 5 min and then the mixture was transferred to a Teflon-lined autoclave and hydrothermally treated at 180 °C for 10 h. Finally, the resulting sample was freeze-dried to obtain the dual-doped porous carbon structure (Scheme 1).2.5. Synthesis of gold nanoparticles immobilized on N and S co-doped porous carbon structure derived from P2AT
Aqueous solution of HAuCl4·3H2O (1 mg in 2.0 mL) and (N, S)-PCM (56 mg in 5.0 mL) were mixed and placed in an ultrasonic bath for 30 min to disperse the metal ions well throughout the nitrogen and sulfur doped carbon structure. Then 4.8 mL of a 0.1 M NaBH4 solution was added to the mixture to reduce and form gold nanoparticles. After stirring for 4 h, it was filtered under vacuum, washed well with ethanol and water and dried under vacuum at 50 °C for 12 h.2.6. General procedure for the catalytic reduction of nitrobenzenes
The reduction of 2-nitroaniline (2-NA) with NaBH4 was carried out as a model reaction to examine the catalytic activity and reusability of the AuNPs@(N, S)-PCM catalyst. Amounts of 1.5 mL of deionized water, 500 μL of 2-NA aqueous solution (2 mM), and 100 μL of fresh NaBH4 (0.2 M) were added into a flask in sequence, followed by addition of 40 μL of catalyst (1 mg mL−1) to the mixture. The progress of the reaction was monitored by tracking the changes in the UV-Vis absorption spectra of the mixture. After completion of the reaction, the catalyst was removed and then reused in the next cycle. The catalytic reduction reactions of other nitrobenzenes were conducted under the same conditions of 2-NA.The catalytic reduction of 2-nitroaniline with H2 was also investigated. For a typical hydrogenation procedure, 0.02 g AuNPs@(N, S)-PCM, 1 mmol 2-NA and 15 mL deionized water were charged in the flask. The hydrogenation took place under magnetic stirring and an atmospheric hydrogen pressure at 30 °C.
3. Results and discussion
3.1. Synthesis and recognition of the catalyst
At first, the polymerization of 2-aminothiazole was accomplished by the rapid mixing method using acetic acid as a dopant and sodium hypochlorite as an oxidant with the special ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for monomer
1 for monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dopant and 2
dopant and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for monomer
1 for monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxidant. To confirm the successive synthesis of P2AT, FT-IR was employed, which is in good accordance with the literature26 (see Fig. S1A†). The morphology of the as-obtained poly(2-aminothiazole) was investigated by SEM as shown in Fig. 1A–C.
oxidant. To confirm the successive synthesis of P2AT, FT-IR was employed, which is in good accordance with the literature26 (see Fig. S1A†). The morphology of the as-obtained poly(2-aminothiazole) was investigated by SEM as shown in Fig. 1A–C.
As can be seen in the SEM image, a special plate structure with a height of approximately 8–10 μm, consisting of nanoparticles, was obtained. According to the Brunauer–Emmett–Teller (BET) method, the surface area of P2AT was calculated to be 38.54 m2 g−1.
Moreover, according to the obtained results from TGA (Fig. 2A), P2AT thermally degrades in four steps. 1.2% weight loss below 100 °C is attributed to loss of moisture, adsorbed solvent or monomer. The major weight loss occurring on 200–600 °C is related to the conversion of P2AT to various subunits including NH3, HCN, CS2 and carbine residue. DTA curve of P2AT is given in Fig. S2.†
After the synthesis and characterization of P2AT, hydrothermal carbonization followed by freeze drying were employed for the synthesis of the nitrogen and sulfur co-doped porous carbon structure. The hydrothermal carbonization reaction was carried out at mild temperature (180 °C) and in pure water inside a sealed autoclave and under self-generated pressure. It has been shown that hydrothermal carbonization leads to more technical and structurally well-defined charring by a controlled chemical method, which justifies its extensive application to produce various carbonaceous materials with attractive structures.
The FT-IR spectrum of the as-obtained nitrogen and sulfur co-doped porous carbon material derived from P2AT is shown in Fig. S1C.† The peaks at 1627 and 614 cm−1 attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–S stretching vibrations, respectively, are observed.
N and C–S stretching vibrations, respectively, are observed.
Morphological study of (N, S)-PCM was accomplished by SEM which is shown in Fig. 1D–F. The pumice-like morphology, including pores inside the pores, ranging from several nanometers to micrometers, can be observed in the SEM images of the synthesized material. One of the most important factors for choosing a material as a support for catalytic application is its surface area. The surface area of (N, S)-PCM was determined by the BET method to be 334.3 m2 g−1. BET testing also showed a mesoporous and macroporous structure with average pore sizes of 2.1 nm from analysis of the adsorption branch by the Barrett–Joyner–Halenda (BJH) method. To investigate the thermal stability of (N, S)-PCM, TGA analysis was performed, which showed that the weight loss starts at 280 °C (Fig. 2B).
In comparison with the XRD pattern of P2AT (Fig. 3A), the hydrothermal carbonization product represents a strong and wide peak at 2θ of 24.7°, assigned to the hexagonal graphite (002) reflection (2θ = 26°).40 The shift to the lower value is due to the presence of sulfur atoms, and can be directly associated with the much larger size of the sulfur atom compared to carbon or nitrogen.41,42 Since nitrogen was also doped, which compresses the layers due to higher polarization interactions and so compensates the effect, the change in position of the (002) peak is lower compared to other sulfur doped reports.
XPS, as one of the most versatile techniques, was employed to investigate the composition of the as-synthesized material and the chemical states of the elements. The XPS spectrum of (N, S)-PCM has been presented in Fig. 4, in which the peaks at 285, 532, 400, and 164 eV, related to C 1s, O 1s, N 1s, and S 2p, are observed, indicating the successful doping of S and N into the porous carbon structure by hydrothermal carbonization of P2AT. The chemical states of C, N and S in the (N, S)-PCM were further studied by high resolution XPS. The high-resolution spectrum of C 1s in (N, S)-PCM can be divided into several single peaks, corresponding to sp2-hybridized carbon atoms bound to neighboring carbon atoms or hydrogen (284.8 eV) and the peaks assigned to the electron-poor carbon bound to nitrogen or sulfur (286.0 eV)43 and –N–C(S)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (288.5 eV),44 further proving that N and S heteroatoms have been doped into the porous carbon structure. The bonding configurations of N and S atoms in the (N, S)-PCM were also investigated based on high-resolution N 1s and S 2p XPS spectra. The N 1s peak in the XPS spectrum can be fitted into three peaks: pyridinic-like N (398.6), pyrrolic-like N (399.9) and graphitic-like N (401.2), which are typically observed in N-doped carbon materials.45 Moreover, the high resolution S 2p peak can be fitted with three different peaks at the binding energies of 164.0 (S 2p3/2) and 164.9 eV (S 2p1/2), attributed to the sulfur binding in C–S bonds and conjugated –C
N– (288.5 eV),44 further proving that N and S heteroatoms have been doped into the porous carbon structure. The bonding configurations of N and S atoms in the (N, S)-PCM were also investigated based on high-resolution N 1s and S 2p XPS spectra. The N 1s peak in the XPS spectrum can be fitted into three peaks: pyridinic-like N (398.6), pyrrolic-like N (399.9) and graphitic-like N (401.2), which are typically observed in N-doped carbon materials.45 Moreover, the high resolution S 2p peak can be fitted with three different peaks at the binding energies of 164.0 (S 2p3/2) and 164.9 eV (S 2p1/2), attributed to the sulfur binding in C–S bonds and conjugated –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S– bonds, and 168.8 eV related to the oxidized sulfur (–SOn–).46
S– bonds, and 168.8 eV related to the oxidized sulfur (–SOn–).46
The proposed precursor for N, S dual-doped carbon material was compared with recently reported precursors in terms of the procedure, heteroatom contents, and surface area, and the results are summarized in Table 1. Elemental analysis (CHN) of (N, S)-PCM shows 55.77% C, 17.36% N, 2.54% H and 12.81% S. In addition, according to the XPS results, carbon, nitrogen, sulfur and oxygen contents are 57.93, 14.2, 4.45, and 23.42 at%, which shows that the (N, S)-PCM displays a high amount of heteroatom-doping, with N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C and S
C and S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratios of up to 1
C ratios of up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13. Among several important reports, our precursor presents much higher amounts of heteroatom doping, which makes it unique for various applications. Moreover, in most cases, high temperatures and complicated processes are required to obtain the N, S co-doped carbon materials, while we use a simple hydrothermal treatment. Actually, employing P2AT as the precursor provides the advantages of a single step reaction with only one compound as a source of N and S under relatively mild conditions, high contents of the heteroatoms, high surface area, and gram-scale product. Therefore, due to the one-pot and straightforward synthesis, high surface area and also the presence of nitrogen and sulfur dual doped in the as-prepared material, it seems that it can be an ideal choice to embed metal nanoparticles specifically for catalytic applications. Furthermore, after the successful synthesis and characterization of (N, S)-PCM, gold nanoparticles were introduced onto the support by the reduction of HAuCl4·3H2O using NaBH4.
13. Among several important reports, our precursor presents much higher amounts of heteroatom doping, which makes it unique for various applications. Moreover, in most cases, high temperatures and complicated processes are required to obtain the N, S co-doped carbon materials, while we use a simple hydrothermal treatment. Actually, employing P2AT as the precursor provides the advantages of a single step reaction with only one compound as a source of N and S under relatively mild conditions, high contents of the heteroatoms, high surface area, and gram-scale product. Therefore, due to the one-pot and straightforward synthesis, high surface area and also the presence of nitrogen and sulfur dual doped in the as-prepared material, it seems that it can be an ideal choice to embed metal nanoparticles specifically for catalytic applications. Furthermore, after the successful synthesis and characterization of (N, S)-PCM, gold nanoparticles were introduced onto the support by the reduction of HAuCl4·3H2O using NaBH4.
| Entry | Precursor for nitrogen and sulfur doping | Procedure | Amount of N-doping/amount of S-doping | Surface area m2 g−1 | Ref. |
|---|---|---|---|---|---|
| 1 | Cyanamide (N-doping), FeSO4·7H2O (S-doping) | Carbonization at 1050 °C | 0.9/0.6 (at%) | — | 47 |
| 2 | Thiophene/acetonitrile | Pyrolysis at 900 °C | 7.6–8.3/0.7–1.6 (at%) | 150–240 | 48 |
| 3 | Cysteine | Pyrolysis at 900 °C | 2.7/2.74 (wt%) | 180.5 | 43 |
| 4 | Thiourea and formaldehyde | Heating at 800 °C | 8.5–17/3–8 (wt%) | 81–850 | 49 |
| 5 | Thiazolium salts: 3-methyl-thiazol-3-ium-dicyanamide and 3-(cyanomethyl)-thiazol-3-iumbromide | Carbonization at 1000 °C | 5.19–6.17/2.17–3.84 (wt%) | 1174 and 1195 | 50 |
| 6 | S-(2-Thienyl)-L-cysteine or 2-thienyl carboxaldehyde | Pyrolysis at 900 °C | 4.3–5/0.74–1.0 (wt%) | 224.5 and 321 | 51 |
| 7 | Melamine (N-doping), benzyl disulfide (S-doping) | Heating at 900 °C | 4.5/2.0 (at%) | 157–220 | 52 |
| 8 | Human hair (keratin containing cysteine) | Hydrothermal treatment at 180 °C, heating at 600 °C | 2.6–3.1/0.2–1.3 (at%) | 849–897 | 53 |
| 9 | SBA-15, sucrose and thiourea | Annealing at 1000 °C | 6.53/2.88 (at%) | 394 | 45 |
| 10 | Citric acid as the carbon source, L-cysteine as nitrogen and sulfur source | Hydrothermal treatment, 200 °C | — | — | 54 |
| 11 | Pyrrole and sulfuric acid | Carbonization at 650–950 °C | 4.8–10/1.7–2.6 (at%) | 978–1021 | 55 |
| 12 | KIT-6 as the template and pyrrole as the precursor | Carbonization at 650–950 °C | 10.0–4.6/0.94–0.75 (at%) | 1064, 693, and 880 depends on temperature | 56 |
| 13 | Graphene oxide, thiourea | Annealing at 700 °C | ∼6.5/0.63 (at%) | — | 57 |
| 14 | Poly(2-aminothiazole) | Hydrothermal treatment, 180 °C | 14.2/4.45 (at%) | 334.3 | This work |
To study the morphology of the (N, S)-PCM after the immobilization of AuNPs, SEM analysis was undertaken, as shown in Fig. 1G and H. As can be seen, the porous structure of the carbon material was retained but its morphology was changed due to the loading of AuNPs on their surfaces.
Transmission electron microscope (TEM) analysis on AuNPs@(N, S)-PCM reveals the synthesis of ultra-small, monodisperse AuNPs with diameters between 1–4 nm on the (N, S)-PCM (Fig. 5). It was expected that due to the presence of both nitrogen and sulfur in the synthesized material, the support could stabilize Au nanoparticles with excellent dispersity.
We further characterized the AuNPs@(N, S)-PCM using XRD (Fig. 3C). (N, S)-PCM shows a broad diffraction peak for C(002) at 2θ = 24.7° and is interpreted in terms of short-range order in stacked carbon sheets, but in AuNPs@(N, S)-PCM the diffraction peak for C(002) is negligible, indicating that significant face-to-face stacking is absent, and suggesting that the carbon sheets were exfoliated due to the loading of AuNPs on their surfaces.58 In addition, two strong bands are observed at 2θ = 37.90° and 44.05°, which correspond to the (111) and (200) crystallographic planes of AuNPs, and two additional peaks appear at 2θ = 64.47° and 77.50°, related to the (220) and (311) crystallographic planes, respectively,59 which is in good agreement with reference to the fcc structure of metallic gold (JCPDS file no. 01-1174). The broadening of Bragg’s peaks shows the formation of nanoparticles. According to the Scherrer equation based on the (111) peak line-width at half-maximum intensity, the average size of AuNPs was estimated to be 2.2 nm, which is in good agreement with TEM results.
The nitrogen adsorption isotherms of P2AT, (N, S)-PCM and AuNPs@(N, S)-PCM and pore-size distributions obtained from adsorption branch using the BJH method have been presented in Fig. S3.† According to the BET measurements of AuNPs@(N, S)-PCM, the surface area of the catalyst with 2.0 nm pore sizes is estimated to be around 174.9 m2 g−1, thus decreasing greatly with Au nanoparticle loading compared to 334.3 m2 g−1 for (N, S)-PCM. This may be caused by a partial blockage of the (N, S)-PCM pores by AuNPs and/or a partial collapse of the mesoporous structure.
3.2. Catalytic activity
The catalytic activity of the as-synthesized gold nanoparticles immobilized on sulfur and nitrogen co-doped porous carbon was investigated by choosing the reduction reaction of 2-nitroaniline with NaBH4 as a model reaction. This reaction, despite being thermodynamically favorable, does not occur spontaneously due to the presence of a kinetic barrier, but in the presence of metal nanoparticles as catalyst, the kinetic barrier was overcome through facilitating electron transfer from the donor BH4− to the acceptor 2-nitroaniline.60 Since 2-nitroaniline displays a typical absorbance peak at 410 nm, which decreases as it is converted to 1,2-benzenediamine, the progress of the reaction could be easily monitored by UV-Vis (Fig. S4†). According to the result, 2-nitroaniline was completely reduced after 12 min.To show the role and performance of the support, and the possible usability of the support as a metal free catalyst, the catalytic reduction reaction of 2-nitroaniline was carried out using (N, S)-PCM under the same reaction conditions. According to the result, the reaction was completed after 56 min, which indicated that the only support can be used as an efficient metal free catalyst for the reduction reactions. In 2013, Kong et al. reported the metal-free catalytic reduction of 4-nitrophenol to 4-aminophenol mediated by N-doped graphene. They suggested that the adsorption and activation of 4-nitrophenol ions only can be carried out by the carbon atoms next to the doped N atoms, due to its weakened conjugation and higher positive charge density.61 In addition, the presence of S atoms in the ((N, S)-PCM) may probably enhance the catalytic effect, due to its small band gap or a metallic form.62 Also, the π–π interactions of the aromatic group with the support prefer the close proximity of the reagents to the catalytic sites and establishing an efficient catalytic reduction.
To reveal the performance of the catalyst, several nitrobenzenes were subjected to the reduction reaction using NaBH4 in the presence of AuNPs@(N, S)-PCM as the catalyst (Table 2, Fig. S5–S8†), whose complete conversion was reflected by the color change of the solution, from originally bright yellow to colorless. Since, in the nitroarene reactions, the formation of diazo side products can significantly decrease the yields, the yields obtained by GC were also presented (Table 2). The presence of substituents such as OH, and NH2 in ortho and para situations leads to the decreased activity towards reduction because of the electron-donating properties. The catalytic reduction of 2-nitroaniline with H2 was also investigated, in which 1,2-benzenediamine was obtained with a yield of 95% after 87 min.
| Entry | Compound | Product | Time (min) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 1.5 mL of deionized water, 500 μL of nitrobenzene aqueous solution (2 mM), and 100 μL of fresh NaBH4 (0.2 M), 40 μL of AuNPs@(N, S)-PCM (1 mg mL−1) at 25 °C. | ||||
| 1 |  |
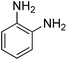 |
12 | 97 |
| 2 |  |
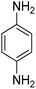 |
9 | 96 |
| 3 |  |
 |
12 | 99 |
| 4 | 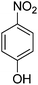 |
 |
58 | 96 |
| 5 |  |
 |
29 | 92 |
The comparison of various AuNP catalysts of 2NA reduction with NaBH4 as a reducing agent is gathered in Table S1.† The as-prepared catalyst indicated good results compared to most of the reported catalysts in terms of mol% Au and time of reaction completion. Moreover, the synthesized support could be successfully employed as a metal free catalyst for catalytic reduction of 2-nitroaniline.
The catalyst stability and recyclability are important issues in the supported catalyst, therefore we investigated the stability and recyclability of the as-synthesized catalyst by measuring the conversions up to six cycles after 4 min for the reduction reaction of 2-nitroaniline as a model reaction (the reaction progress was monitored by GC). The results are summarized in Fig. S9,† proving the high stability and recyclability of the catalyst. Additionally, the leaching of the catalyst was investigated, in which, interestingly, no leaching of the active metal occurred, due to the high stability of Au nanoparticles.
4. Conclusion
In this report, for the first time, the synthesis of P2AT with special morphology was presented by a rapid mixing method. Subsequently, P2AT was used as a single source of nitrogen and sulfur to obtain N, S-co doped porous carbon by a simple hydrothermal carbonization reaction followed by freeze drying. The SEM and BET results showed the high surface area and porosity of the synthesized material, which makes it suitable as a support for catalytic applications. After the synthesis of (N, S)-PCM, very small and monodisperse gold nanoparticles were immobilized on it, as proven by TEM and XRD analyses. The AuNPs@(N, S)-PCM exhibited high activity toward the reduction reaction of nitrobenzenes.Moreover, the applicability of the support as a metal free catalyst was evaluated for catalytic reduction of 2-nitroaniline, showing very good results.
Acknowledgements
We are grateful to Shahid Beheshti University Research Council for partial financial support of this work.Notes and references
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760–764 CrossRef CAS PubMed.
- S. Wang, D. Yu and L. Dai, J. Am. Chem. Soc., 2011, 133, 5182–5185 CrossRef CAS PubMed.
- I. Kruusenberg, N. Alexeyeva, K. Tammeveski, J. Kozlova, L. Matisen, V. Sammelselg, J. Solla-Gullon and J. M. Feliu, Carbon, 2011, 49, 4031–4039 CrossRef CAS PubMed.
- Y. Li, W. Zhou, H. Wang, L. Xie, Y. Liang, F. Wei, J.-C. Idrobo, S. J. Pennycook and H. Dai, Nat. Nanotechnol., 2012, 7, 394–400 CrossRef CAS PubMed.
- D. Yu, E. Nagelli, F. Du and L. Dai, J. Phys. Chem. Lett., 2010, 1, 2165–2173 CrossRef CAS.
- P. Zhang, J. Lian and Q. Jiang, Phys. Chem. Chem. Phys., 2012, 14, 11715–11723 RSC.
- N. Alexeyeva, E. Shulga, V. Kisand, I. Kink and K. Tammeveski, J. Electroanal. Chem., 2010, 648, 169–175 CrossRef CAS PubMed.
- G. P. Hao, G. Mondin, Z. Zheng, T. Biemelt, S. Klosz, R. Schubel, A. Eychmüller and S. Kaskel, Angew. Chem., Int. Ed., 2015, 54, 1941–1945 CrossRef CAS PubMed.
- N. Fechler, T.-P. Fellinger and M. Antonietti, J. Mater. Chem. A, 2013, 1, 14097–14102 CAS.
- Y. Zheng, Y. Jiao, M. Jaroniec, Y. Jin and S. Z. Qiao, Small, 2012, 8, 3550–3566 CrossRef CAS PubMed.
- J. Kouvetakis, R. B. Kaner, M. L. Sattler and N. Bartlett, J. Chem. Soc., Chem. Commun., 1986, 1758–1759 RSC.
- Q. Shi, F. Peng, S. Liao, H. Wang, H. Yu, Z. Liu, B. Zhang and D. Su, J. Mater. Chem. A, 2013, 1, 14853–14857 CAS.
- D. Yu, Y. Xue and L. Dai, J. Phys. Chem. Lett., 2012, 3, 2863–2870 CrossRef CAS.
- S. Wang, E. Iyyamperumal, A. Roy, Y. Xue, D. Yu and L. Dai, Angew. Chem., Int. Ed., 2011, 50, 11756–11760 CrossRef CAS PubMed.
- J. P. Paraknowitsch and A. Thomas, Energy Environ. Sci., 2013, 6, 2839–2855 CAS.
- J. Liang, Y. Jiao, M. Jaroniec and S. Qiao, Angew. Chem., Int. Ed., 2012, 51, 11496–11500 CrossRef CAS PubMed.
- W. Shen and W. Fan, J. Mater. Chem. A, 2013, 1, 999–1013 CAS.
- J. P. Paraknowitsch and A. Thomas, Macromol. Chem. Phys., 2012, 213, 1132–1145 CrossRef CAS PubMed.
- J. Yuan, A. G. Marquez, J. Reinacher, C. Giordano, J. Janek and M. Antonietti, Polym. Chem., 2011, 2, 1654–1657 RSC.
- J. P. Paraknowitsch, J. Zhang, D. Su, A. Thomas and M. Antonietti, Adv. Mater., 2010, 22, 87–92 CrossRef CAS PubMed.
- M. R. Nabid, Y. Bide and Z. Habibi, RSC Adv., 2015, 5, 2258–2265 RSC.
- M. R. Nabid, Y. Bide and M. Abuali, RSC Adv., 2014, 4, 35844–35851 RSC.
- J. Cruz, E. Garcia-Ochoa and M. Castro, J. Electrochem. Soc., 2003, 150, B26–B35 CrossRef CAS PubMed.
- A. Cukurovalı, İ. Yılmaz, M. Ahmedzade and S. Kırbağ, Heteroat. Chem., 2001, 12, 665–670 CrossRef PubMed.
- X. Zhou, L. Shao, Z. Jin, J. B. Liu, H. Dai and J. X. Fang, Heteroat. Chem., 2007, 18, 55–59 CrossRef CAS PubMed.
- M. Bıyıkoğlu and H. Çiftçi, Polym. Bull., 2013, 70, 2843–2856 CrossRef.
- M. Yıldırım and İ. Kaya, Synth. Met., 2012, 162, 436–443 CrossRef PubMed.
- J. Huang and R. B. Kaner, Angew. Chem., Int. Ed., 2004, 43, 5817–5821 CrossRef CAS PubMed.
- S. J. T. Rezaei, Y. Bide and M. R. Nabid, Synth. Met., 2011, 161, 1414–1419 CrossRef CAS PubMed.
- M. Reza Nabid, S. J. Tabatabaei Rezaei and S. Zahra Hosseini, Mater. Lett., 2012, 84, 128–131 CrossRef CAS PubMed.
- Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239–3265 CrossRef CAS PubMed.
- A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096–2126 RSC.
- M. R. Nabid, Y. Bide and S. J. Tabatabaei Rezaei, Appl. Catal., A, 2011, 406, 124–132 CrossRef CAS PubMed.
- M. R. Nabid, Y. Bide and M. Niknezhad, ChemCatChem, 2014, 6, 538–546 CrossRef CAS PubMed.
- M. R. Nabid and Y. Bide, Appl. Catal., A, 2014, 469, 183–190 CrossRef CAS PubMed.
- M. R. Nabid, Y. Bide, E. Aghaghafari and S. Rezaei, Catal. Lett., 2014, 144, 355–363 CrossRef CAS.
- M. R. Nabid, Y. Bide, N. Ghalavand and M. Niknezhad, Appl. Organomet. Chem., 2014, 28, 389–395 CrossRef CAS PubMed.
- S. S. Muir and X. Yao, Int. J. Hydrogen Energy, 2011, 36, 5983–5997 CrossRef CAS PubMed.
- O. Ozay, E. Inger, N. Aktas and N. Sahiner, Int. J. Hydrogen Energy, 2011, 36, 8209–8216 CrossRef CAS PubMed.
- Z. Li, C. Lu, Z. Xia, Y. Zhou and Z. Luo, Carbon, 2007, 45, 1686–1695 CrossRef CAS PubMed.
- S. Glenis, A. Nelson and M. Labes, J. Appl. Phys., 1999, 86, 4464–4466 CrossRef CAS PubMed.
- Y. Wu, S. Fang, Y. Jiang and R. Holze, J. Power Sources, 2002, 108, 245–249 CrossRef CAS.
- C. H. Choi, S. H. Park and S. I. Woo, Green Chem., 2011, 13, 406–412 RSC.
- N. Baccile, M. Antonietti and M.-M. Titirici, ChemSusChem, 2010, 3, 246–253 CrossRef CAS PubMed.
- Z. Liu, H. Nie, Z. Yang, J. Zhang, Z. Jin, Y. Lu, Z. Xiao and S. Huang, Nanoscale, 2013, 5, 3283–3288 RSC.
- S. Yang, L. Zhi, K. Tang, X. Feng, J. Maier and K. Müllen, Adv. Funct. Mater., 2012, 22, 3634–3640 CrossRef CAS PubMed.
- H. T. Chung, C. M. Johnston, K. Artyushkova, M. Ferrandon, D. J. Myers and P. Zelenay, Electrochem. Commun., 2010, 12, 1792–1795 CrossRef CAS PubMed.
- E. J. Biddinger, D. S. Knapke, D. von Deak and U. S. Ozkan, Appl. Catal., B, 2010, 96, 72–82 CrossRef CAS PubMed.
- T. Tsubota, K. Takenaka, N. Murakami and T. Ohno, J. Power Sources, 2011, 196, 10455–10460 CrossRef CAS PubMed.
- J. P. Paraknowitsch, B. Wienert, Y. Zhang and A. Thomas, Chem.–Eur. J., 2012, 18, 15416–15423 CrossRef CAS PubMed.
- S.-A. Wohlgemuth, R. J. White, M.-G. Willinger, M.-M. Titirici and M. Antonietti, Green Chem., 2012, 14, 1515–1523 RSC.
- J. Liang, Y. Jiao, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2012, 51, 11496–11500 CrossRef CAS PubMed.
- W. Si, J. Zhou, S. Zhang, S. Li, W. Xing and S. Zhuo, Electrochim. Acta, 2013, 107, 397–405 CrossRef CAS PubMed.
- Y. Dong, H. Pang, H. B. Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed., 2013, 52, 7800–7804 CrossRef CAS PubMed.
- D. Zhang, Y. Hao, L. Zheng, Y. Ma, H. Feng and H. Luo, J. Mater. Chem. A, 2013, 1, 7584–7591 CAS.
- D. Zhang, L. Zheng, Y. Ma, L. Lei, Q. Li, Y. Li, H. Luo, H. Feng and Y. Hao, ACS Appl. Mater. Interfaces, 2014, 6, 2657–2665 CAS.
- X. Wang, J. Wang, D. Wang, S. Dou, Z. Ma, J. Wu, L. Tao, A. Shen, C. Ouyang and Q. Liu, Chem. Commun., 2014, 50, 4839–4842 RSC.
- Y. Si and E. T. Samulski, Chem. Mater., 2008, 20, 6792–6797 CrossRef CAS.
- M. J. Rak, N. K. Saadé, T. Friščić and A. Moores, Green Chem., 2014, 16, 86–89 RSC.
- J. Huang, L. Zhang, B. Chen, N. Ji, F. Chen, Y. Zhang and Z. Zhang, Nanoscale, 2010, 2, 2733–2738 RSC.
- X.-K. Kong, Z.-Y. Sun, M. Chen and Q.-W. Chen, Energy Environ. Sci., 2013, 6, 3260–3266 CAS.
- P. A. Denis, R. Faccio and A. W. Mombru, ChemPhysChem, 2009, 10, 715–722 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09272h |
| This journal is © The Royal Society of Chemistry 2015 |






