Bioactive prodigiosin-impregnated cellulose matrix for the removal of pathogenic bacteria from aqueous solution
Abstract
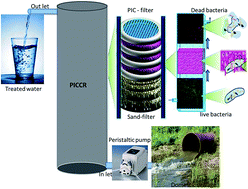
The increase in concern over safe water for human consumption demands disinfection of water. Conventional chemical disinfection methods release counter ions into the treated water and they impair human health significantly. The present investigation was focused on the disinfection of pathogenic bacteria via a bio-disinfection route using a prodigiosin-impregnated cellulose column reactor (PICCR). Escherichia coli and Bacillus cereus were chosen as model pathogens to validate the efficiency of the PICCR for pathogen removal from water. The pathogen removal efficiency was evaluated by the pour plate method, regrowth ability in nutrient broth and quantitative estimation of live and dead cells using fluorescent microscopy. The PICCR showed effective reduction of E. coli by 97.31% and B. cereus by 97.33%. Further, bacterial cell membrane damage by bio-disinfection was verified through analysis of the residual protein and nucleic acid in the treated water using UV-visible spectroscopy, a trans-illuminometer and SDS PAGE. The proposed PICCR was found to be effective for the removal of pathogens from water and this may be regarded as a viable purification technique for drinking water.

 Please wait while we load your content...
Please wait while we load your content...