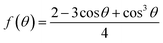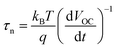A large grain size perovskite thin film with a dense structure for planar heterojunction solar cells via spray deposition under ambient conditions†
Zhurong Liangab,
Shaohong Zhanga,
Xueqing Xu*ab,
Nan Wanga,
Junxia Wanga,
Xin Wanga,
Zhuoneng Biab,
Gang Xuab,
Ningyi Yuanc and
Jianning Dingc
aGuangzhou Institute of Energy Conversion, Renewable Energy and Gas Hydrate Key Laboratory of Chinese Academy of Sciences, Guangzhou 510640, China. E-mail: xuxq@ms.giec.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cJiangsu Collaborative Innovation Center of Photovoltaic Science and Engineering, Changzhou University, Jiangsu 213164, China
First published on 7th July 2015
Abstract
Organometal halide perovskites have emerged as promising light absorbers for third-generation photovoltaics. Herein we developed a facile spray deposition process to prepare high-quality perovskite films without any post-annealing under ambient conditions with a high humidity of up to 50%. The as-prepared perovskite films exhibit large grain sizes up to micrometers and full surface coverage. These desirable features significantly enhance the light harvesting efficiency and reduce charge recombination. Furthermore, the morphology and film thickness can be easily controlled by varying the precursor concentration or scanning times during spray deposition. The as-fabricated planar heterojunction solar cells with an optimized perovskite film thickness exhibited a power conversion efficiency of ∼7.89%, which is expected to be further improved with the increase of substrate temperature, the utilization of more compatible substrates, and the optimization of the hole-transport layer and device structure. This simple low-temperature manufacturing process provides a novel strategy for the scalable and fast fabrication of high-quality absorber layers for efficient perovskite based solar cells. The film formation mechanism regarding the nucleation and growth of perovskite films with desirable morphology is also discussed.
Introduction
Organometal halide perovskites have emerged as competitive light absorbers in solar cells with a rapid improvement in efficiency from 3.8% in 2009 to 20.1% in 2014,1,2 which is comparable to state-of-the-art copper indium gallium diselenide (CIGS) solar cells.3 The unprecedented achievement in power conversion efficiency (PCE) of perovskite solar cells is attributed to the remarkable properties of these materials, such as appropriate direct bandgap, high absorption coefficient, long exciton diffusion lengths, and excellent carrier mobility.4–8 Highly efficient perovskite solar cells with mesoporous TiO2 as electron contact, in analogy to typical dye-sensitized solar cells (DSSCs), or with Al2O3 as insulating scaffold have been demonstrated to date.9 The utilization of mesoporous layers, to some extent, promotes film formation and improves surface coverage. However, complementary to these mesoporous structured devices, perovskite solar cells in a planar geometry, without any scaffold, have attracted great interest owing to the simpler structure, and low-temperature processing, which is suitable for flexible devices.In planar heterojunction perovskite solar cells with a sandwich configuration, photocarriers need to transfer through the perovskite layer. The crystallinity, morphology, thickness, and surface coverage of this absorber film are therefore expected to be critical to the device performance.10 To avoid the shunting in such device structure, numerous methods were explored to prepared high-quality perovskite layer, such as improved spin-coating,11,12 two-step sequential deposition,13,14 vacuum vapor deposition,15 and so on. In a conventional one-step spin-coating method, perovskite layers are gradually formed with the volatilization of solvent during spinning and further crystallized in a post-annealing process. However, films produced by this method were found to be of poor surface coverage with island-like or dendritic perovskite, which induced a huge amount of pin-hole areas and thereby greatly reduce the cell performance. To address these issues, on the one hand, additive-induced strategies were explored by quickly adding an anti-solvent during the spin-coating process, in which the additive can rapidly reduce the solubility of perovskite and remove the excess solvent, leading to the fast nucleation and growth of dense perovskite crystal layer.11,16 On the other hand, two-step sequential deposition was also demonstrated as an alternative route to prepare uniform perovskite films with homogenous structure and full surface coverage,14 but it suffers from incomplete conversion from PbI2 to perovskite and involves longer manufacturing time.13 Vacuum vapor deposition is an effective route to obtain extremely uniform and pin-hole free perovskite layer. Perovskite solar cells with planar structure based on these techniques have achieved high PCE up to 12–15%.15,17,18 Despite the promising results, vapor deposition will greatly increase the processing cost and not suitable for large-scale manufacturing compared with the solution processes. Alternative routes with simple procedures and low cost are therefore desired to prepare high-quality perovskite films. Yang and co-workers reported a low-temperature vapor-assisted solution process (VASP) to obtain polycrystalline perovskite layer with full surface coverage and large grain size, in which the as-deposited PbI2 film was further annealed in the present of CH3NH3I vapor at around 150 °C in N2 atmosphere and finally converted to perovskite thin film.19 Though the as-fabricated solar cells achieved an impressive PCE of 12.1%, the fabrication process is relatively complex and needs to be performed under inert condition.
Recently, Lidzey et al. has reported the deposition of CH3NH3PbI3−xClx perovskite films on the poly(3,4-ethylenedioxythiophene)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(styrene sulfonate) (PEDOT
poly(styrene sulfonate) (PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS) coated indium tin oxide (ITO) substrate held at 75 °C using ultra-sonic spray-coating method under ambient condition.20 The optimal films are characterized by a hierarchy structures on a micron length-scale, and the surface coverage of perovskite on the substrate takes a maximum value of ∼85%. The resulted solar cells with PEDOT
PSS) coated indium tin oxide (ITO) substrate held at 75 °C using ultra-sonic spray-coating method under ambient condition.20 The optimal films are characterized by a hierarchy structures on a micron length-scale, and the surface coverage of perovskite on the substrate takes a maximum value of ∼85%. The resulted solar cells with PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS and [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) as electrode interfacial layers owned an average PCE of ∼7.8% and a maximum PCE of ∼11%. Most recently, Nie et al. reported a solution-based hot-casting method to grow high-quality continuous perovskite with millimeter-scale grains and sufficient coverage, where a hot (∼70 °C) mixture of lead iodide (PbI2) and methylamine hydrochloride (MACl) solution was casted onto a substrate maintained at a temperature of up to 180 °C and subsequently spin coated to obtain a uniform film.21 It is observed that the grain size significantly increased from approximately 20 to 180 μm with the increase of the substrate temperature from 100 to 190 °C, and the resulted solar cells obtained PCE from 5% to 18%.
PSS and [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) as electrode interfacial layers owned an average PCE of ∼7.8% and a maximum PCE of ∼11%. Most recently, Nie et al. reported a solution-based hot-casting method to grow high-quality continuous perovskite with millimeter-scale grains and sufficient coverage, where a hot (∼70 °C) mixture of lead iodide (PbI2) and methylamine hydrochloride (MACl) solution was casted onto a substrate maintained at a temperature of up to 180 °C and subsequently spin coated to obtain a uniform film.21 It is observed that the grain size significantly increased from approximately 20 to 180 μm with the increase of the substrate temperature from 100 to 190 °C, and the resulted solar cells obtained PCE from 5% to 18%.
In this work, we propose a scalable spray deposition method to fabricate planar heterojunction perovskite solar cells, in which the perovskite solution were directly sprayed onto the TiO2 substrates held at 110 °C under ambient atmosphere with humidity higher than 50%. A dense perovskite layer with continuous large grain size up to 2–3 micrometer and full surface coverage were immediately formed during the spray deposition process without any post-annealing. The successful formation of high-quality perovskite thin film are attributed to the abundant nucleation and epitaxial growth of perovskite crystals. The as-fabricated planar heterojunction solar cells with an optimized perovskite films thickness exhibited a PCE of ∼7.89%. The film formation mechanism and the correlation between perovskite and substrate regarding the nucleation and growth of perovskite films with desirable morphology are discussed as well.
Experimental
Synthesis of CH3NH3I
Methyl ammonium iodide (CH3NH3I) was synthesized according to the reported procedure.22 In a typical synthesis, a mixture of 10 ml hydroiodic acid (55.0–58.0% in water, Aladdin) and 9.8 ml methylamine (30–33 wt% in absolute ethanol, Aladdin) was stirred in a 50 ml round-bottomed flask at 0 °C for 2 hours. The resulting solution was recovered by evaporation at 50 °C for 1 h. The precipitate was dissolved in ethanol, recrystallized from diethyl ether, and dried at 60 °C in a vacuum oven for 24 h.Device fabrication
The fluorine-doped tin oxide (FTO)-coated glass substrates were patterned by etching with zinc powder and hydrochloric acid, followed by washing with soap, deionized water, ethanol, and isopropanol. The substrates were dried with compressed nitrogen and treated with ozone before use. The TiO2 compact layer was deposited by spray pyrolysis at 450 °C of the precursor solution, titanium(IV) bis(acetoacetonato)di (isopropanoxylate) (98%, Sigma-Aldrich) diluted in ethanol. To obtain CH3NH3PbI3 solution, the synthesized CH3NH3I powder was mixed with PbI2 (99.9%, Aladdin) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in 4 ml anhydrous N,N-dimethylformamide (DMF), and stirred at room temperature for 20 min to produce a clear CH3NH3PbI3 solution (11.3 wt%), followed by filtering with a 0.45 μm PTFE filter. CH3NH3PbI3 solution with concentration of 15.0 wt%, 9.0 wt%, and 5.6 wt% were prepared in a similar manner. To deposit perovskite films, the TiO2-coated FTO substrates were held on the hotplate (SCHOTT Instruments) at 110 °C beforehand. The perovskite solution were then sprayed onto the substrates by with a spray gun (Prona RH-C Air Brush, Taiwan) using nitrogen as the carrier gas. All spraying procedures were carried out under ambient atmosphere at an invariant scan rate of 10 cm s−1. Note that the nozzle height was essential to the perovskite film quality and fixed at 10 cm from the substrates. The colour of the substrates immediately changed to black once the perovskite solution was sprayed on it. The hole-transport material (HTM) solution was spin-coated on the CH3NH3PbI3 perovskite layer at 4000 rpm for 30 s, which was prepared by dissolving 72.3 mg of 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamine)-9,9-spirobifluorene (spiro-OMeTAD), 28.8 μl of 4-tert-butylpyridine (>96.0%, TCI), and 17.5 μl of lithiumbis (trifluoromethanesulfonyl) imide (Li-TFSI) (99.95%, Sigma-Aldrich) solution (520 mg Li-TFSI in 1 ml acetonitrile, 99.8%, Sigma-Aldrich) in 1 ml of chlorobenzene. Finally, 150 nm of gold was magnetron sputtered as the cathode on the spiro-OMeTAD-coated film.
1 molar ratio in 4 ml anhydrous N,N-dimethylformamide (DMF), and stirred at room temperature for 20 min to produce a clear CH3NH3PbI3 solution (11.3 wt%), followed by filtering with a 0.45 μm PTFE filter. CH3NH3PbI3 solution with concentration of 15.0 wt%, 9.0 wt%, and 5.6 wt% were prepared in a similar manner. To deposit perovskite films, the TiO2-coated FTO substrates were held on the hotplate (SCHOTT Instruments) at 110 °C beforehand. The perovskite solution were then sprayed onto the substrates by with a spray gun (Prona RH-C Air Brush, Taiwan) using nitrogen as the carrier gas. All spraying procedures were carried out under ambient atmosphere at an invariant scan rate of 10 cm s−1. Note that the nozzle height was essential to the perovskite film quality and fixed at 10 cm from the substrates. The colour of the substrates immediately changed to black once the perovskite solution was sprayed on it. The hole-transport material (HTM) solution was spin-coated on the CH3NH3PbI3 perovskite layer at 4000 rpm for 30 s, which was prepared by dissolving 72.3 mg of 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenylamine)-9,9-spirobifluorene (spiro-OMeTAD), 28.8 μl of 4-tert-butylpyridine (>96.0%, TCI), and 17.5 μl of lithiumbis (trifluoromethanesulfonyl) imide (Li-TFSI) (99.95%, Sigma-Aldrich) solution (520 mg Li-TFSI in 1 ml acetonitrile, 99.8%, Sigma-Aldrich) in 1 ml of chlorobenzene. Finally, 150 nm of gold was magnetron sputtered as the cathode on the spiro-OMeTAD-coated film.
Characterization
The X-ray diffraction (XRD) patterns of the perovskite were measured on X'pert Pro MPD X-ray diffractometer using Cu Kα irradiation at a scan rate (2θ) of 0.0167° S−1. The accelerating voltage and applied current were 40 kV and 40 mA, respectively. Raman spectra of the perovskite films were detected on LabRAM HR800 with the excitation wavelength of 532 nm. Morphology and microstructural characterization were performed using an S-4800 High resolution field emission scanning electron microscope (FESEM) (Hitachi, Japan). The diffuse reflection and transmission spectra of the perovskite films were measured on a PerkinElmer Lambda 750 UV/VIS/NIR spectrophotometer. The absorption spectra of the samples were obtained according to absorption (%) = 100% − transmittance (T) − reflectance (R). The current density–voltage (J–V) curves were measured using an Autolab TYPE II electrochemical work station. The cells were illuminated using the ABET Sun 3000 solar simulator with a source meter (Keithley 2420) at 100 mA cm−2 illumination (AM 1.5G), where the light intensity was adjusted with an NREL calibrated silicon solar cell. All the measurements of the solar cells were performed under ambient atmosphere at room temperature without any encapsulation.Results and discussion
The CH3NH3PbI3 films were prepared via our facile spray deposition method with different concentration of precursor solutions under ambient condition with humidity higher than 50%. The XRD pattern of the spray-deposited perovskite films are shown in Fig. 1a. Intense diffraction peaks at 14.02°, 19.98°, 23.38°, 24.42°, 28.38°, 31.83°, 34.92°, 40.53°, and 43.08° can be respectively assigned to (110), (112), (211), (202), (220), (310), (312), (224), and (314) crystal planes of the tetragonal perovskite phase.23 The sharp and intense diffraction peaks of (110) and (220) indicate a highly oriented crystal structure with large grain size and enhanced crystallinity. Furthermore, the intensity of the diffraction peaks of (110) and (220) increased with the concentration of the precursor solutions, which implies the increase of the grain size perpendicular to these crystal planes. The absence of the peak at 12.65° (the (001) diffraction peak for PbI2) in perovskite films obtained using precursor solutions with concentration of 9.0 wt%, 11.3 wt%, and 15.0 wt% indicating a high level of phase purity of the perovskite films. When utilizing a low concentration precursor solution of 5.6 wt%, PbI2 was detected, which could be attributed to the insufficient crystallization of CH3NH3PbI3. In order to obtain a more precise assignment of the perovskite structure, Raman spectra measurement was performed with a perovskite film prepared using a precursor solution with a concentration of 11.3 wt%. As shown in Fig. 1b, the sharp bands at 70 and 95 cm−1 mainly corresponding to the bending and stretching of the Pb–I bonds respectively, which are the characteristic modes of the inorganic cage in the perovskite crystal structure. The bands falling at 107 and 136 cm−1 can be associated to the vibrations of the CH3NH3+ cations. The bands at approximately 241 and 347 cm−1 should be assigned to the torsional modes of the methylammonium cation.24 On the basis of the Raman analysis, the as-prepared perovskite herein is tetragonal structures with high crystallinity, which is consistent with the XRD results.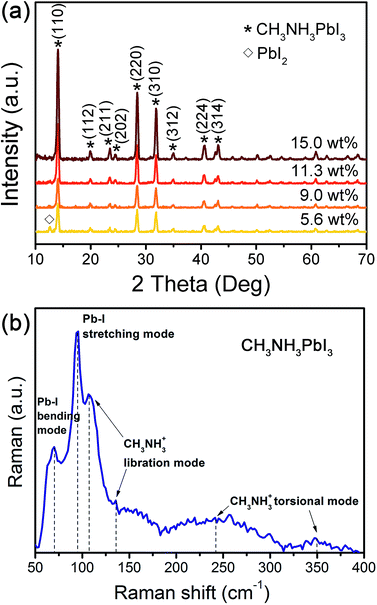 | ||
| Fig. 1 (a) XRD patterns, and (b) Raman spectra of the perovskite film prepared via spray deposition method. | ||
Morphology of the perovskite films prepared with different concentration of precursor solutions were observed using a FESEM as shown in Fig. 2, and the relevant grain size distributions were estimated as listed in Table 1. With fixed 2 times of spraying, grain sizes of the perovskite films prepared using different perovskite precursor concentrations, i.e. 5.6 wt%, 9.0 wt%, 11.3 wt%, and 15.0 wt%, were 321 ± 101 nm, 542 ± 158 nm, 759 ± 200 nm, and 1139 ± 451 nm, respectively. It can be observed that relatively low precursor concentration, e.g. 5.6 wt%, 9.0 wt%, are correlated with undesirable perovskite films having incomplete surface coverage (Fig. 2a and b). It can be imagined that precursor solutions with low concentrations could not supply enough precursor species for the growth of the grains leading to large intervals between the grains and hence incomplete surface coverage. On the other hand, it is found that low concentration of precursor solutions will cause more serious heat-lose of the substrate when the droplets fall on it, leading to the decrease of substrate temperature, which is unfavorable for the further evaporation of the solvents resulted in the disturbance of the liquid films. By increasing the precursor concentration to 11.3 wt%, and 15.0 wt%, the perovskite films exhibit full surface coverage and small surface roughness (Fig. 2c and d). Additionally, the average grain size of the perovskite films increases greatly. However, it is important to note that, by increasing the concentration of precursor solutions, the viscosities of the solutions also increase, which will reduce the wetting properties (Fig. S1† presents the contact angles of the droplet with different concentration of precursor solutions on compact TiO2) and pulverization of the solutions, which might lead to uneven films. In another word, a suitable concentration of precursor solution is critical to the formation of uniform perovskite films with large grain size.
| Perovskite solution concentration | Thickness (nm) | Average grain size (nm) | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) | Best PCE (%) |
|---|---|---|---|---|---|---|---|
| a All the photovoltaic parameters are the average of a batch of four devices. | |||||||
| 5.6 wt% | 248 ± 7 | 321 ± 101 | 10.77 | 0.79 | 0.50 | 4.06 ± 0.2 | 4.25 |
| 9.0 wt% | 331 ± 3 | 542 ± 158 | 14.96 | 0.80 | 0.44 | 4.78 ± 0.4 | 5.24 |
| 11.3 wt% | 484 ± 11 | 759 ± 200 | 18.81 | 0.87 | 0.48 | 7.40 ± 0.4 | 7.89 |
| 15.0 wt% | 670 ± 17 | 1139 ± 451 | 16.67 | 0.82 | 0.44 | 5.51 ± 0.3 | 5.96 |
On the other hand, we also prepared a series of perovskite films using precursor solution with an invariant concentration (11.3 wt%) by varying the scanning times during spray deposition and systematically investigated their morphologies (Fig. 3). Interestingly, the thickness of the perovskite films increases almost linearly with the increase of the scanning times (Fig. 4), and meanwhile the grain size increased greatly with the scanning times. The cross sectional SEM image for 4 times of spraying presents a perfect large grain in size up to 2–3 micrometers. In comparison of perovskite thin film deposited by spin-coating and spray deposition, the FESEM images of films deposited by spin-coating were also measured and shown in Fig. S2.† It can be seen that the perovskite films obtained by spin-coating are dendritic shape with small grain size and large pin-hole areas.
 | ||
| Fig. 4 Film thickness versus scanning time for the perovskite films prepared via the spray deposition method using a precursor solution with an invariant concentration of 11.3 wt%. | ||
Based on the sufficient investigation of SEM images of the perovskite films during spray deposition, a deep insight was given into the nucleation and growth process, and the schematic procedure can be divided into several stages as illustrated in Fig. 5a. Firstly, precursor solution consisting of PbI2, CH3NH3I, and DMF are sprayed from the nozzle and spread as numerous droplets, and at the mean time the solvent continuously evaporates during this procedure. When the droplets fall on the hot substrate, the solution rapidly reach the supersaturation with the evaporation of solvent resulting in the nucleation and growth of perovskite grains. With the continuous deposition of the perovskite droplets, the precursor species diffuse onto the surface of the seed grains leading to the further growth of perovskite. Finally, the perovskite crystals grow into continuous thermodynamically stable films with large grain size and full surface coverage.
The morphology of the films are related with the interface and surface free energies. At the first stage, the nucleation process occurred on the surface or foreign nuclei is a typical heterogeneous nucleation as shown in Fig. 5b, the free energy of which (ΔGheterogeneous) can be expressed as follows:25
| ΔGheterogeneous = ΔGhomogeneous × f(θ) |
and
where σLN is the interface free energy between the nuclei and the liquid, σLS is the interface free energy between the substrate and the liquid, and σNS is the interface free energy between the substrate and the nuclei. It can be seen that the decrease of σNS will cause the decrease of the contact angle (θ), which in turn reduces the nucleation barrier, leading to the fast nucleation of perovskite and growth into a continuous film. Especially, if the perovskite and the substrate have a perfect lattice match, i.e. σNS approaches to zero and σLN ≈ σLS, the contact angle θ and ΔGhomogeneous will tend to be zero, which will facilitate the nucleation and growth of perovskite films (Fig. 5c). In this sense, related study on finding different compatible substrates are desirable in the future. It is worth noting that in the second stage, the perovskite precursor solutions are continuously sprayed onto the seed grains resulting in the epitaxial growth of perovskite crystals, which leads to large grain size and sufficient coverages. Apart from the crystal match between nuclei and substrate, the wetting properties of precursor solution on substrate are also significant to the formation of uniform films. The contact angles of perovskite precursor solution in DMF with different concentrations on the surface of various substrates are shown in Fig. S1.† It is indicated that the contact angles of the precursor solutions on the TiO2 substrate are smaller than that on the PEDOT:PSS substrate, which is beneficial to the formation of uniform films. In addition, polarity of solvent, temperature of substrate,26 and other factors affecting the wetting properties will affect the contact angle of the droplets and hence the morphology of the films, which need further investigations.
The UV-vis absorption spectra of the perovskite films prepared using different precursor concentrations are shown in Fig. 7a, in which all films exhibit absorption onsets at 800 nm. The light absorption intensity gradually enhances with the increase of the precursor concentration, owing to the improvement of the surface coverage and increase of the perovskite film thickness. In addition, the red-shift of the absorption edge should be attributed to the improved crystallinity and/or the full reaction of precursors during the film formation process.27 Therefore, for samples obtained utilizing a relatively higher precursor concentration of 11.3 wt% or 15.0 wt%, an excellent light absorption almost above 80% in visible region was observed. The different perovskite films were fabricated as absorption layers in planar heterojunction solar cells with a typical sandwich configuration (Fig. 6a and b). The photocurrent density–voltage (J–V) curves of the solar cells were recorded, the corresponding cell performance sweeping from open-circuit to short-circuit under the forward bias voltage (named as reverse scan) with scan rate of 0.05 V s−1 are presented in Fig. 7b, and the corresponding photovoltaic parameters of which are summarized in Table 1. It can be seen that, when the precursor concentration increases from 5.6 to 11.3 wt%, both of the short-circuit current density (Jsc) and open-circuit voltage (Voc) significantly increase owing to the enhancement of the light absorption resulted from the improvement of the crystallinity and surface coverage of the perovskite films. It is important to note that, with a same perovskite film thickness (∼450 nm), the Jsc obtained in this work (18.81 mA cm−2) is higher than that obtained in the previous reported ultra-sonic spray-deposition method (10.4 mA cm−2),20 which indicated that the perovskite film quality herein is much better. Whereas, the device incorporating a perovskite layer prepared from 15.0 wt% precursor concentration exhibited a lower Jsc, Voc, fill factor (FF), and PCE as respected to that from ∼11.3 wt%, which can be attributed to the over-thickness of the perovskite films. It is reported that perovskite solar cells exhibit hysteresis behaviors in J–V curves, especially the solar cells with a planar structure. We also observed similar behaviors in our planar solar cells (Fig. S3†), in which the reverse scan curves show a lower recombination current than the forward scan curves. For comparisons, we also prepared perovskite films on the mesoporous TiO2 and related solar cells. It was observed that the J–V curves of the solar cells with a mesoporous structure exhibits a very small hysteresis effect compared with the planar solar cells (Fig. S4†), which is in good agreement with the common reported results.16,28,29 This hysteresis behaviors could be attributed to large density of defect states within/near the surface of the perovskite films, or due to the ferroelectric properties of the perovskite materials.28,30 Besides, the destruction of the HTM layers during the magnetron sputtering process for the gold electrodes in this work may slow down the extraction of the holes and lead to severe charge recombination especially during the forward scan of the J–V curves. In addition, the HTM layers in this work also need to be further optimized in terms of the material purity, solution concentration, layer thickness, and dopant levels. Anyway, the exact reasons for the hysteresis behaviors need to be further investigated. On the other hand, we also investigated the different photovoltaic performance between the devices utilizing the spray deposited and spin-coated perovskite films (Fig. S5†). It is observed that the device with spray deposited perovskite films has a better performance than the control device, which is mainly attributed to the improved morphology of the perovskite films (Fig. S2†).
 | ||
| Fig. 6 (a) Schematic illustration, and (b) cross sectional SEM image of a planar heterojunction perovskite solar cell. | ||
Photovoltage decay measurements were performed to investigate the charge recombination properties of the as-fabricated perovskite solar cells (Fig. 7c). The electron lifetime (τn) could be derived from the photovoltage decay curve according to the following formula,31
Conclusions
In conclusion, we have developed a facile spray deposition process to prepare high-quality perovskite films without any post-annealing under ambient condition and high humidity up to 50%. The as-prepared perovskite films exhibit full surface coverage, uniform grain structure with grain size up to 2–3 micrometers. The as-fabricated planar heterojunction solar cells utilizing a 484 ± 11 nm perovskite film exhibited a PCE of ∼7.89% (Jsc = 18.81 mA cm−2, Voc = 0.87 V, and FF = 48%). The grain size and morphology of the perovskite films are expected to be further improved with the increase of substrate temperature and the utilization of more compatible substrates. Moreover, further study on HTM layer and device structure optimization to enhance the fill factor and overall device performance is underway. It is envisioned that this simple low-temperature manufacturing process will be a novel strategy to the scalable and fast production of desirable absorber layers for efficient perovskite based solar cells.Acknowledgements
This work was supported by the National Natural Science Foundation of China (projects 21073193, 21273241), Science and Technology Research Project of Guangzhou City (2014J4100218), Project on the Collaborative Innovation and Environmental Construction Platform of Guangdong Province (2014A050503051), and Project of Jiangsu Province Industry-University-Research Joint Innovation Fund (BY2013024-01).Notes and references
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed
.
- D. Shi, V. Adinolfi, R. Comin, M. Yuan, E. Alarousu, A. Buin, Y. Chen, S. Hoogland, A. Rothenberger, K. Katsiev, Y. Losovyj, X. Zhang, P. A. Dowben, O. F. Mohammed, E. H. Sargent and O. M. Bakr, Science, 2015, 347, 519–522 CrossRef CAS PubMed
.
- R. F. Service, Science, 2014, 344, 458 CrossRef PubMed
.
- C. Wehrenfennig, G. E. Eperon, M. B. Johnston, H. J. Snaith and L. M. Herz, Adv. Mater., 2014, 26, 1584–1589 CrossRef CAS PubMed
.
- T. Baikie, Y. Fang, J. M. Kadro, M. Schreyer, F. Wei, S. G. Mhaisalkar, M. Graetzel and T. J. White, J. Mater. Chem. A, 2013, 1, 5628–5641 CAS
.
- C. S. Ponseca Jr, T. J. Savenije, M. Abdellah, K. Zheng, A. Yartsev, T. Pascher, T. Harlang, P. Chabera, T. Pullerits, A. Stepanov, J.-P. Wolf and V. Sundstrom, J. Am. Chem. Soc., 2014, 136, 5189–5192 CrossRef PubMed
.
- G. E. E. S. D. Stranks, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–343 CrossRef PubMed
.
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Graetzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 342, 344–347 CrossRef CAS PubMed
.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed
.
- Y. Zhao and K. Zhu, J. Phys. Chem. Lett., 2014, 5, 4175–4186 CrossRef CAS
.
- M. Xiao, F. Huang, W. Huang, Y. Dkhissi, Y. Zhu, J. Etheridge, A. Gray-Weale, U. Bach, Y.-B. Cheng and L. Spiccia, Angew. Chem., Int. Ed., 2014, 53, 9898–9903 CrossRef CAS PubMed
.
- F. Huang, Y. Dkhissi, W. Huang, M. Xiao, I. Benesperi, S. Rubanov, Y. Zhu, X. Lin, L. Jiang, Y. Zhou, A. Gray-Weale, J. Etheridge, C. R. McNeill, R. A. Caruso, U. Bach, L. Spiccia and Y.-B. Cheng, Nano Energy, 2014, 10, 10–18 CrossRef CAS PubMed
.
- Y. Wu, A. Islam, X. Yang, C. Qin, J. Liu, K. Zhang, W. Peng and L. Han, Energy Environ. Sci., 2014, 7, 2934–2938 CAS
.
- J. Burschka, N. Pellet, S. J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Gratzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed
.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed
.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and I. S. Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed
.
- C.-W. Chen, H.-W. Kang, S.-Y. Hsiao, P.-F. Yang, K.-M. Chiang and H.-W. Lin, Adv. Mater., 2014, 26, 6647–6652 CrossRef CAS PubMed
.
- O. Malinkiewicz, A. Yella, Y. H. Lee, G. M. Espallargas, M. Graetzel, M. K. Nazeeruddin and H. J. Bolink, Nat. Photonics, 2013, 8, 128–132 CrossRef PubMed
.
- Q. Chen, H. Zhou, Z. Hong, S. Luo, H.-S. Duan, H.-H. Wang, Y. Liu, G. Li and Y. Yang, J. Am. Chem. Soc., 2014, 136, 622–625 CrossRef CAS PubMed
.
- A. T. Barrows, A. J. Pearson, C. K. Kwak, A. D. F. Dunbar, A. R. Buckley and D. G. Lidzey, Energy Environ. Sci., 2014, 7, 2944–2950 CAS
.
- W. Nie, H. Tsai, R. Asadpour, J.-C. Blancon, A. J. Neukirch, G. Gupta, J. J. Crochet, M. Chhowalla, S. Tretiak, M. A. Alam, H.-L. Wang and A. D. Mohite, Science, 2015, 347, 522–525 CrossRef CAS PubMed
.
- J. H. Heo, S. H. Im, J. H. Noh, T. N. Mandal, C.-S. Lim, J. A. Chang, Y. H. Lee, H.-j. Kim, A. Sarkar, M. K. Nazeeruddin, M. Grätzel and S. I. Seok, Nat. Photonics, 2013, 7, 486–491 CrossRef CAS PubMed
.
- Y. Xiao, G. Han, Y. Li, M. Li and J. Wu, J. Mater. Chem. A, 2014, 2, 16856–16862 CAS
.
- C. Quarti, G. Grancini, E. Mosconi, P. Bruno, J. M. Ball, M. M. Lee, H. J. Snaith, A. Petrozza and F. De Angelis, J. Phys. Chem. Lett., 2014, 5, 279–284 CrossRef CAS
.
- T. Salim, S. Sun, Y. Abe, A. Krishna, A. C. Grimsdale and Y. M. Lam, J. Mater. Chem. A, 2014, 3, 8943–8969 Search PubMed
.
- M. Saliba, K. W. Tan, H. Sai, D. T. Moore, T. Scott, W. Zhang, L. A. Estroff, U. Wiesner and H. J. Snaith, J. Phys. Chem. C, 2014, 118, 17171–17177 CAS
.
- Z. Xiao, Q. Dong, C. Bi, Y. Shao, Y. Yuan and J. Huang, Adv. Mater., 2014, 26, 6503–6509 CrossRef CAS PubMed
.
- H. J. Snaith, A. Abate, J. M. Ball, G. E. Eperon, T. Leijtens, N. K. Noel, S. D. Stranks, J. T.-W. Wang, K. Wojciechowski and W. Zhang, J. Phys. Chem. Lett., 2014, 5, 1511–1515 CrossRef CAS
.
- H.-S. Kim and N.-G. Park, J. Phys. Chem. Lett., 2014, 5, 2927–2934 CrossRef CAS
.
- H.-S. Kim, I. Mora-Sero, V. Gonzalez-Pedro, F. Fabregat-Santiago, E. J. Juarez-Perez, N.-G. Park and J. Bisquert, Nat. Commun., 2013, 4, 2242 Search PubMed
.
- A. Zaban, M. Greenshtein and J. Bisquert, ChemPhysChem, 2003, 4, 859–864 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Contact angles of perovskite precursor solution with different concentration on the surfaces of different substrates; surface SEM images of the perovskite thin films prepared by spray deposition and spin-coating; photocurrent density–voltage (J–V) at forward scan and reverse scan for planar solar cells and mesoporous solar cells using perovskite films by spray deposition; J–V curves for solar cells employed perovskite films prepared using a precursor solution concentration of 11.3 wt% by spray deposition, and spin-coating. See DOI: 10.1039/c5ra09110a |
| This journal is © The Royal Society of Chemistry 2015 |