Colorimetric detection of Al(iii) in vermicelli samples based on ionic liquid group coated gold nanoparticles†
Abstract
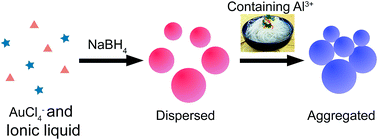
We synthesized ionic liquid (1-ethyl-3-methylimidazolium thiocyanate)-coated gold nanoparticles (IL-Au NPs) using a one-pot method and introduced them in the colorimetric assay for Al3+. IL-Au NPs showed good sensitivity and selectivity. They can determine the concentration of aluminum in vermicelli samples.

 Please wait while we load your content...
Please wait while we load your content...