Multifunctionalities of near-infrared upconversion luminescence, optical temperature sensing and long persistent luminescence in La3Ga5GeO14:Cr3+,Yb3+,Er3+ and their potential coupling†
Xiong Yi,
Zitao Chen,
Shi Ye*,
Ye Li,
Enhai Song and
Qinyuan Zhang
State Key Lab of Luminescent Materials and Devices, Guangdong Engineering Technology Research and Development Center of Special Optical Fiber Materials and Device, Guangdong Provincial Key Laboratory of Fiber Laser Materials and Applied Techniques, South China University of Technology, Guangzhou 510641, China. E-mail: msyes@scut.edu.cn
First published on 28th May 2015
Abstract
The multifunctionalities of La3Ga5GeO14:Cr3+,Yb3+,Er3+ (LGG:Cr3+,Yb3+,Er3+) with respective functions of near-infrared (NIR) upconversion (UC) luminescence, optical temperature sensing (OTS) and long persistent luminescence (LPL) were carried out and investigated in detail, which makes the materials attractive for bioapplications. The NIR UC luminescence at ∼830 nm with deep penetration in tissues is ascribed to the 4T2 → 4A2 transition of Cr3+. The OTS function based on the intensity ratio variation of 2H11/2 → 4I15/2 to 4S3/2 → 4I15/2 transitions of Er3+ ion detected that the thermal effects of the UC material caused by the laser irradiation ranges from 307 to 332 K for pumping power of 60 to 86 mW mm−2. It also showed LPL of Cr3+ with a defect trap depth of ∼0.77 eV below the conduction band, benefiting excitation-free and noise-free imaging in tissues. Additionally, anomalous temperature dependant UC emission behaviours of Cr3+ and Er3+ in this material were also characterized and discussed, which can be understood by the configurational coordinate (CC) model and the complex forward and backward energy transfer processes among the dopants, respectively. The potential coupling of these functions was further discussed, such as UC induced NIR LPL, which would repetitively restrengthen the fading LPL signals and alert the operators to thermal damage of the biosystems by the NIR laser in the tissue imaging.
I. Introduction
Coupled multifunctionalities would result in novel functional materials with potential applications in some specific fields, for instance, luminescent materials with multi-modes in biosystems. UC luminescence is a process in which long-wavelength (low energy) photons are converted into a shorter wavelength (high energy) photon.1 Great attention has been paid to UC luminescent materials due to their potential applications in various areas such as color displays, medical, biological labels, anti-counterfeiting, solar cells and so on.2–5 Specially, the research of NIR UC luminescent materials is essential for optical probes in biosystems because of the low background noise and deep tissue penetration compared to visible UC materials.6–8 Tm3+ is the most commonly used NIR emitting ion in the Yb3+ codoped UC materials.6,9,10 However, owing to the well shield 4f electrons by outer shell 5s5p electrons, the tunability of luminescence and multifunctionality for the lanthanide ions are deficient. Whereas, transition metal (TM) ions have the complementally virtues and might be adopted in NIR UC luminescent materials. It was recently reported that Mn2+ could show anomalous NIR UC emission in KZnF3:Yb3+,Mn2+ and MgGa2O4:Yb3+,Mn2+.11,12 Besides the Mn2+, Cr3+ is another known NIR emitting TM ion and Yb3+–Cr3+ is a potential efficient NIR UC system.13–16LPL is a process with luminescence last for minutes to hours at room temperature after the stoppage of the excitation.17 The NIR LPL is extremely attractive in biolabels for its excitation-free and noise-free imaging conditions.18 Again, Cr3+ is the most potential NIR LPL ion with outstanding performance.19–21 It is recently reported that the gallogermanate La3Ga5GeO14:Cr3+ exhibits attractive NIR LPL property.22
Additionally, pumping the UC materials by the high power laser in order to get strong fluorescence signals in the bioapplications would inevitably cause temperature rise of the materials.23,24 It should be necessary and possible to incorporate the OTS function in the UC system to detect the thermal temperature. Yb3+–Er3+ is the most commonly used ions for OTS owing to that the intensity ratio of 2H11/2 → 4I15/2 to 4S3/2 → 4I15/2 transitions of Er3+ ion is independent of luminescence loss and fluctuations in excitation intensity.25–27 Overall, codoping Er3+ in Yb3+–Cr3+ UC system might realize the multifunctionalities of NIR UC emission, OTS and LPL. In addition, the host of La3Ga5GeO14 (LGG) offers proper sites for accommodating the Cr3+ ions and Yb3+/Er3+ ions for triply doping since the radius of Cr3+(coordination number CN = 6), Ga3+ (CN = 6), Yb3+ (CN = 8), Er3+ (CN = 8) and La3+ (CN = 8) are 0.615 Å, 0.62 Å, 0.985 Å, 1.004 Å and 1.16 Å, respectively.28 In the structure of the host, Ge4+ and partial Ga3+ are formed by (Ga/Ge)O4 tetrahedrons and GaO4 tetrahedrons with coordination of four, while some of Ga3+ are formed by GaO6 octahedra with coordination of six. The energy transfer processes among these dopants were detailedly investigated in our previous work.29 Hence, we are convinced that this La3Ga5GeO14:Cr3+,Yb3+,Er3+ material could fulfill the multifunctionalities of NIR UC emission, OTS and LPL. Further, we attempt to give an insight to the temperature dependant luminescence behaviours and couple these functions to accomplish some more novel function.
II. Experimental
The LGG:xCr3+,yYb3+,zEr3+ (short for La1−y−zGa5−xGeO14:xCr3+,yYb3+,zEr3+) phosphors were synthesized by a solid-state reaction method as reported in our previous work.29 Typically, stoichiometric amounts of starting materials La2O3 (99.99%), Ga2O3 (analytical reagent, A.R.), GeO2 (99.99%), Cr2O3 (A.R.), Yb2O3 (99.99%), Er2O3 (99.99%) and NH4Cl (A.R., 10 wt%) were weighted and mixed thoroughly in an agate mortar. NH4Cl was added as flux. Then the mixture was sequentially fired at 1273 K and 1573 K for 4 h with intermediate grindings.Phase purity was checked by PANalytical X' pert Pro X-ray diffractometer with Cu anode target (Kα1 = 1.54059 Å). XRD data for refinement was collected on a Bruker D8 ADVANCE X-ray diffractometer with Cu target and Ni filter. The step-scan mode was used with setup of 40 kV × 40 mA, step 0.01°, 0.2 s per step. Rietveld refinement was conducted by TOPAS-Academic program. UC emission spectra were recorded on the Jobin-Yvon TRIAX320 spectrofluorimeter equipped with a R928 photomultiplier tube as the detector and a 976 nm laser diode (LD, Coherent Corp.) as monochromatic light source. To study temperature dependant behaviour of the UC luminescence, the same spectrofluorometer was equipped with a TAP-02 high-temperature fluorescence accessory (Tian Jin Orient–KOJI instrument Co., Ltd.). Thermo-luminescence glow curves were measured with a FJ-427A TL meter (Beijing Nuclear Instrument Factory) by heating the irradiated samples from 313 to 473 K. The samples were pre-irradiated by using a simulative sunlight lamp for 5 min and then heated at a linear rate of 2 K s−1 to release the energy reserved in the material. For testing the thermo-luminescence (TL) curves with laser, the sample was excited by a 976 nm laser for 5 min before testing. LPL decay curve was tested on a long afterglow Material Optical Test System (Hangzhou, Everfine corporation), and the sample was irradiated by 365 nm laser for 10 min before testing.
III. Results and discussion
3.1 XRD patterns and crystal structure
Fig. 1 is the X-ray powder diffraction patterns of La3Ga5GeO14 and some typical samples La2.82Ga5−xGeO14:xCr3+,0.12Yb3+,0.06Er3+ (x = 0.15, 0.30). It can be observed that all the samples are in agreement with ICSD #20783, except for the sample of La0.82Ga4.7GeO14:0.30Cr3+,0.12Yb3+,0.06Er3+ with an obvious impurity phase of LaGaO3 (JCPDS 54-870, marked with inverted triangle). It clearly suggests that Cr3+, Yb3+ and Er3+ ions have been successfully incorporated into the host lattice for those samples with dopant contents less than that of La0.82Ga4.7GeO14:0.30Cr3+,0.12Yb3+,0.06Er3+. Reitveld refinement graph of LGG sample is illustrated in Fig. 2 and some of the refinement results are listed in Table 1. LGG has a space group of P321, its 2d site is disorderly occupied by 50% of Ga(2) and 50% of Ge. Layers formed by (Ga/Ge)O4 tetrahedrons and GaO4 tetrahedrons sharing the corner are connected by GaO6 octahedra along c axis, with La3+ ions in the forming cages of the framework (as seen in Fig. 3). Some typical cation–cation distances are also listed in Table 1. It should be noted that the distance between La–Ga is shorter than that of La–La. | ||
| Fig. 1 XRD patterns of some typical samples (La1−y−zGa5−xGeO14:xCr3+,yYb3+,zEr3+; LGG:CYE), the inverted triangle shows an impurity phase. | ||
| Cation–cation distance (Å) | |||||
|---|---|---|---|---|---|
| a Space group: P321 (no. 150), Z = 1, V = 297.80(9) Å3, a = 8.2049(6) Å, c = 5.1080(4) Å, Rwp = 6.89%. | |||||
| La–Ga1 | 3.4387 | La–Ga2 | 3.4391 | La–Ga3 | 3.8209 |
| La–Ga2/Ge | 3.6556 | La–Ga3 | 3.9288 | ||
| La–La | 5.9560 | La–La | 5.1090 | La–La | 4.2598 |
 | ||
| Fig. 3 Crystal structure of LGG sample (upper, view along a axis); cation surroundings and cation–cation distances (lower, anion is omitted for clarity). | ||
3.2 NIR UC luminescence spectra
Fig. 4 shows the UC emission spectrum of LGG:0.15Cr3+,0.12Yb3+,0.06Er3+ at room temperature (RT) pumped by a 976 nm laser diode (∼60 mW mm−2). It can be observed that all the spectra exhibit four main emission peaks centered at 524, 548, 650, and 830 nm, which can be ascribed to the 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transition of Er3+and 4T2 → 4A2 transition of Cr3+, respectively. Notably, the intense NIR UC emission peak at ∼830 nm of Cr3+ is gained in this LGG material, which is highly desired for bioimaging. According to our previous work,29 detailed energy transfer processes among Yb3+, Er3+ and Cr3+ were discussed and there exist forward and backward energy transfer processes. Our previous work also reveals that both the UC processes of Er3+ and Cr3+ are the two-photon processes.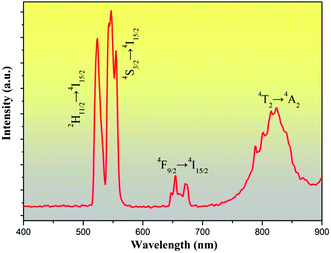 | ||
| Fig. 4 UC luminescence spectra of LGG:015Cr3+,0.12Yb3+,0.06Er3+ upon 976 nm laser diode excitation at RT (∼60 mW mm−2). | ||
3.3 OTS and temperature dependent NIR UC behaviour
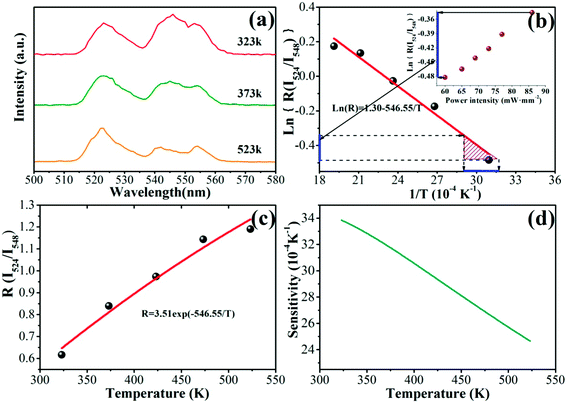
Yb3+–Er3+ doped phosphors can be used as temperature sensors for the reasons as follow.26 The energy gap between the 2H11/2 and 4S3/2 levels is about 840 cm−1 according to the green UC emissions spectra. This energy separation allows the 2H11/2 level populated from 4S3/2 level by thermal agitation, leading to variation in the transitions of 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 of Er3+ at different temperature. With the thermalization of population at the two levels and ignoring the effects of self-absorption of the fluorescence, the fluorescence intensity ratio (FIR) of the two green UC emissions can be written as eqn (1):30
 | (1) |
Fig. 5(a) depicts the green UC emissions spectra for LGG:0.15Cr3+,0.12Yb3+,0.06Er3+ at the temperature of 323, 373 and 523 K. Obviously, the peak locations of two emissions show little change with temperature, but the FIR of the 524 and 548 nm emission peaks varied. Fig. 5(b) illustrates a monolog plot of the FIR of 524 and 548 nm UC emission peaks as a function of inverse absolute temperature in the range of 323–573 K. The slope of the fitting line is about 546.55 according to the experimental data. Then, the FIR of these two peaks relative to the temperature in the range of 323–523 K was shown in Fig. 5(c). The experimental data are fitted to exponential curve with the coefficient C value of 3.51 in eqn (1). The sensitivity of the sensor can be defined as eqn (2):30
 | (2) |
The corresponding resultant curve is described in Fig. 5(d). At the temperature of 323 K, the sensitivity of LGG:0.15Cr3+,0.12Yb3+,0.06Er3+is about 0.0034 K−1. And the sensitivity deceased to 0.0025 K−1 as temperature rises.
The insert of Fig. 5(b) shows a plot of the FIR of the green UC emissions at 524 and 548 nm as a function of power density. As the power density increases, the Ln(R) varied from approximate −0.483 to −0.346. As we known, NIR laser could lead to thermal release.23,24 This variation can be related to thermal temperature, as blue line shown, the corresponding temperature was ranging from 307 to 332 K for pumping power from 60 to 86 mW mm−2.
OTS is an important feature of this material, and for most luminescence materials and devices, their application temperature are usually over 300 K.31 Thus, it needs to investigate the NIR UC behaviours of the phosphors above RT due to that there may be thermal quenching phenomenon and additional energy transfer process in these triply doped phosphors. Therefore, the temperature-dependent UC emission of LGG:xCr3+,0.12Yb3+,0.06Er3+(x = 0.02, 0.04, 0.06, 0.1, 0.15, 0.30) were investigated.
Fig. 6(a, c and e) shows the typical spectra of LGG:xCr3+,0.12Yb3+,0.06Er3+(x = 0.02, 0.06, and 0.15) at temperature from 323 to 573 K. The integrated intensity of different peaks (510 to 570 nm for green emission of Er3+, 640 to 690 nm for red emission of Er3+, 730 to 870 nm for NIR emission of Cr3+) with different concentration of Cr3+ at various temperature were presented in Fig. 6(b, d and f). The other samples can be seen in the ESI (Fig. S1†). Interestingly, the NIR emission intensities of all samples show a rise up firstly then decline with the increase of temperature while that of green emission of Er3+ behave distinctively for samples with different concentration of Cr3+. For the sample with low concentration (x = 0.02) of Cr3+ (Fig. 6(b)), it decreases as temperature rises; while for the sample with higher concentration (x = 0.04 and 0.06) of Cr3+ (Fig. 6(d) and S1(b)†), it show distinct increase first and then decrease as temperature rises; and for the sample with large concentration (x = 0.1, 0.15 and 0.30) of Cr3+ (Fig. 6(f) and S1(d and f)†), it varies little with different temperature. Additionally, the red emission intensities of all samples do not vary dramatically with temperature. Normally, thermal quenching behaviour is expected as the temperature arises because the probabilities of multi-phonon relaxation and energy transfer for quenching the emitting levels are enhanced.32,33 However, anti-thermal-quenching behavior was observed in this system as seen in Fig. 6 and S1.† A possible reason for this phenomenon is that there may exist thermo-luminescence in this system.34 With temperature increases, the photons may be released due to thermal disturbance, so the intensity of luminescence may arise firstly. This is convinced by the fact that LGG:Cr3+ phosphor possesses LPL characteristic.22 Thus, the interpretation of temperature-dependent of UC emission will be discussed after the characterization of LPL behavior.
3.4 LPL behaviour
To find out whether the Yb3+–Er3+–Cr3+ triply doped LGG behaves LPL, thermo-luminescence of the samples were carried out and a typical result of LGG:0.15Cr3+,0.12Yb3+,0.06Er3+ is illustrated in Fig. 7. After heated (black pentacle dots in Fig. 7), it shows a horizontal line at the temperature ranging 313 to 473 K. When the sample was excited by 976 nm laser for 5 min again in a black box, the TL spectrum still appear with a horizontal line as shown in Fig. 7 (red prismatic dots). The TL peak shows up when the sample exposure to the stimulated sunlight for 5 min. These facts suggest that the anomalous temperature dependent UC emissions cannot be ascribed to the LPL of these phosphors since the excitation of 976 nm LD laser on the samples could not lead to any TL peaks. The recovering ability of the temperature-dependent UC spectra of the samples (see that of a typical sample in Fig. S2†) also reveal that there is no TL effect in the temperature-dependent UC process. However, the LGG:0.15Cr3+,0.12Yb3+,0.06Er3+ phosphor indeed possess the LPL function with afterglow time of 19.6 min (5% of luminance left) according to the TL curves in the insert of Fig. 7. The energy depth of the defects that trapped the photo-excited electrons is about 0.77 eV following the eqn (3):35
 | (3) |
3.5 Luminescence models and the potential coupled multifunctionality
Upon a 976 nm laser excitation at RT, Yb3+ ion firstly transfer the absorbed 976 nm photon energy to Er3+ ions via energy transfer process and populate the 4I11/2 level. Then, the Er3+ ion absorbed another 976 nm photon from excitation laser or from energy transfer of Yb3+ to populate the 4F7/2 of Er3+ ions. The Er3+ first relax nonradiatively to the 2H11/2, resulting in the 2H11/2 → 4I15/2 emission. Sequentially, the 4S3/2 level is populated by partial energy relaxation from the 2H11/2 level, producing the 4S3/2 → 4I15/2 emission. Alternatively, it can populate the 4F9/2 through further relaxation, leading to the red emission of 4F9/2 → 4I15/2. As for NIR UC of Cr3+, an cooperation sensitized upconversion is proposed as a three-ion process, in which two excited Yb3+ ions might simultaneously transfer their absorbed energy to a neighboring Cr3+ ion.14 Since the anomalous temperature-dependent UC behaviour can not be caused by TL, it should be the essential attribute of luminescent centers itself in this material. Then we attempt to interpret the abnormal temperature-dependent NIR UC emission of Cr3+ by the configurational coordinate (CC) diagram, as illustrated in Fig. 8. Upon the 976 nm laser excitation, the Yb3+ ions transfer the absorbed two 976 nm photons' energy to Cr3+ ions, resulting in population of 4T1 level from R0 point according to the Frank–Condon principle. Then the electrons at 4T1 level first relax to the equilibrium state of the 4T1 level (point R1) and subsequently relax nonradiatively to the 4T2 level (green dash line, process I), when the electron returns to the equilibrium state (point R2), it would result in 830 nm emission via process II (green line), which is dominative emission at RT. As the temperature rises, thermal activated electrons are pumped from 4T2 level to a higher sublevel 4T2′ with the assistance of phonons in LGG host and then electrons return to the ground state via process IV (pink line). It is noticed that the energy barrier ΔE (the energy difference between the crossing point S and the bottom of the excited state) becomes larger (ΔE2 > ΔE1). As a result, the probability of nonradiative transition across S2 decreases at elevated temperature.36–38 However, when the temperature is getting higher, the vibrational energy makes the excited electrons to reach to the S2 point and then relax to the ground state nonradiatively along the route of R2′ → S2 → R0, resulting in the thermal quenching of luminescence. This can interpret why the NIR emission intensity of Cr3+ goes up firstly and then decline in Fig. 6(b, d and f), (circle pink dots) and Fig. S1(b, d and f),† (circle pink dots).However, the luminescence intensity behaves distinctly for Er3+. Compared with the d–d transitions of TM ions, the f–f transitions of RE ions with sharp line shape of the emission peaks are insensitive to the local chemical environment. Hence the temperature dependent UC behaviour of Er3+ could not be well understood by configurational coordinate. According to our previous work,29 there are several forward and backward energy transfer processes in LGG:Cr3+,Yb3+,Er3+, in which Cr3+ → Yb3+ energy transfer is efficient and Yb3+ acts as an imperative bridge that makes transfer energy from Cr3+ to Er3+ efficient. For x = 0.02, the green UC emission of Er3+ declines monotonously with temperature, which might be due to the normal thermal quenching. Then it behaves analogously to that of NIR UC emission of Cr3+ when the Cr3+ concentration x is 0.04 and 0.06, which is ascribed to the backward energy transfer from Cr3+ (4T1 level) to Er3+ via Yb3+ bridge (2F5/2 level) (as demonstrated in Fig. 9, blue solid arrow). While for Cr3+ concentration x higher than 0.06, the changeless of the green UC emission could be contributed by that larger possibility of forward energy transfer from Yb3+ to Cr3+ than that from Yb3+ to Er3+. This can be deduced by the fact that the distance between Yb3+ (La3+) and Cr3+ (Ga3+) is significantly shorter than that between Yb3+ (La3+) and Er3+ (La3+) and more possibility for Cr3+ (Ga3+) to show up around Yb3+ (La3+), as demonstrated in Fig. 3 and Table 1. Whereas, the invariant red UC emission of Er3+ for samples with x < 0.06 may be owed to the dissipation of red emission through backward transfer to Cr3+ (blue dashed line), based on the fact that the emission of 4F9/2 → 4I15/2 of Er3+ fully overlaps with the 4A2–4T2 absorption of Cr3+.29
For LPL process (as shown in Fig. 9), a high energy photon (UV light)22 excites an electron of Cr3+ from ground state 4A2 to conduction band (CB) of LGG, then, the electrons delocalize to be trapped by the defects. With the thermal activation, the trapped electron is activated to CB then relax to 4T2 level, and finally back to the ground state of 4A2 with NIR emission.
In summary, the luminescence models of LGG:Cr3+,Yb3+,Er3+ with multifunctionality of NIR UC, OTS and LPL is schematically illustrated in Fig. 9. It should be very interesting if coupling these function together, e.g., if the NIR LPL, NIR UC emission and OTS are caused by a single pumping source of 976 nm laser, as the orange dotted curved arrows depicted in Fig. 9. It would make such material rather attractive for bioapplications, since the fading NIR LPL signals in deep tissues could be restrengthened repetitively through NIR laser irradiation. The problem is that the 976 nm laser could not cause delocalized electrons that would be trapped by the defects in this system, which is essential for the thermal perturbation induced luminescence. One possible reason may be that the pumping power of the laser is not high enough to cause delocalized electrons, or in other words, the band gap of LGG is too large. Considering the application of such materials, the pumping power should not be as high as possible. Therefore, choosing the host with narrow band gap and Er3+ ions with proper energy levels below and above conduction band involving in the UC process should be preferential options. Of course the depth of the controllable defects should be another significant factor to be considered. Furthermore, the choice of Er3+ ion in such material with OTS function would alert to prevent the biosystem from thermal damage caused by the intensive laser irradiation.
IV. Conclusions
In conclusion, we have investigated the mutifunctionalities of LGG:Cr3+,Yb3+,Er3+ with respective functions of strong NIR UC, OTS and LPL. NIR UC and LPL are ascribed to Cr3+ emission, while OTS is based on the transition characteristic of Er3+. The laser induced thermal effect would be ranging from 307 to 332 K for pumping power of 60 to 86 mW mm−2 incident on the UC material according to OTS function. The temperature-dependent UC spectra demonstrated that the UC emission intensity of Cr3+ was quite different from that of Er3+, which can be interpreted by the CC model for the former and forward/backward energy transfer processes for the latter. If the LPL could be stimulated by the 976 nm laser, all the three functions would coupling together. It would make such kind of materials with the capacities of repetitively restrengthen the fading LPL signals and alerting the operators to thermal damage of the biosystems in the deep tissue imaging.Acknowledgements
This work is financially joint supported by the NSFC (Grant no. 51125005, 21101065), Outstanding Young Teacher Training Program of Guangdong provincial Institute of higher education (Yq2013011) and Guangdong Natural Science Funds for Distinguished Young Scholar (2014A030306009).References
- F. Auzel, Chem. Rev., 2004, 104, 139 CrossRef CAS PubMed.
- R. Deng, F. Qin, R. Chen, W. Huang, M. Hong and X. Liu, Nat. Nanotechnol., 2015, 10, 273 Search PubMed.
- M. Wang, G. Abbineni, A. Clevenger, C. Mao and S. Xu, Nanomedicine: NBM, 2011, 7, 710 CrossRef CAS PubMed.
- Y. Liu, K. Ai and L. Lu, Nanoscale, 2011, 3, 4804 RSC.
- S. Fischer, J. Goldschmidt, P. Löper, G. Bauer, R. Brüggemann, K. Krämer, D. Biner, M. Hermle and S. Glunz, J. Appl. Phys., 2010, 108, 044912 CrossRef PubMed.
- M. Nyk, R. Kumar, T. Y. Ohulchanskyy, E. J. Bergey and P. N. Prasad, Nano Lett., 2008, 8, 3834 CrossRef CAS PubMed.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976 RSC.
- R. Kumar, M. Nyk, T. Y. Ohulchanskyy, C. A. Flask and P. N. Prasad, Adv. Funct. Mater., 2009, 19, 853 CrossRef CAS PubMed.
- H. T. Wong, H. L. W. Chan and J. Hao, Opt. Express, 2010, 18, 6123 CrossRef CAS PubMed.
- G. Chen, T. Y. Ohulchanskyy, R. Kumar, H. Ågren and P. N. Prasad, ACS Nano, 2010, 4, 3163 CrossRef CAS PubMed.
- E. Song, S. Ding, M. Wu, S. Ye, F. Xiao, S. Zhou and Q. Zhang, Adv. Opt. Mater., 2014, 2, 670 CrossRef CAS PubMed.
- E. Song, J. Wang, D. Yu, S. Ye and Q. Zhang, J. Mater. Chem. C, 2014, 2, 8811 RSC.
- S. Heer, M. Wermuth, K. Krämer and H. Güdel, Chem. Phys. Lett., 2001, 334, 293 CrossRef CAS.
- S. Heer, M. Wermuth, K. Krämer and H. Güdel, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 125112 CrossRef.
- S. Heer, K. Petermann and H. Güdel, J. Lumin., 2003, 102, 144 CrossRef.
- D. Chen, Y. Chen, H. Lu and Z. Ji, Inorg. Chem., 2014, 53, 8638 CrossRef CAS PubMed.
- J. Hölsä, Electrochem. Soc. Interface, 2009, 18, 42 Search PubMed.
- Q. L. M. de Chermont, C. Chanéac, J. Seguin, F. Pellé, S. Maîtrejean, J. P. Jolivet, D. Gourier, M. Bessodes and D. Scherman, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 9266 CrossRef PubMed.
- B. Struve and G. Huber, Appl. Phys. B, 1985, 36, 195 CrossRef.
- L. S. Forster, Chem. Rev., 1990, 90, 331 CrossRef CAS.
- Z. Pan, Y. Y. Lu and F. Liu, Nat. Mater., 2012, 11, 58 CrossRef CAS PubMed.
- W. Yan, F. Liu, Y. Y. Lu, X. J. Wang, M. Yin and Z. Pan, Opt. Express, 2010, 18, 20215 CrossRef CAS PubMed.
- J. Wang, T. Ming, Z. Jin, J. Wang, L. D. Sun and C. H. Yan, Nat. Commun., 2014, 5, 5669 CrossRef CAS PubMed.
- B. Dong, S. Xu, J. Sun, S. Bi, D. Li, X. Bai, Y. Wang, L. Wang and H. Song, J. Mater. Chem., 2011, 21, 6193 RSC.
- B. Dong, B. Cao, Z. Feng, X. Wang and Y. He, Sens. Actuators, B, 2012, 165, 34 CrossRef CAS PubMed.
- B. Dong, B. Cao, Y. He, Z. Liu, Z. Li and Z. Feng, Adv. Mater., 2012, 24, 1987 CrossRef CAS PubMed.
- P. Du, L. Luo, W. Li, Q. Yue and H. Chen, Appl. Phys. Lett., 2014, 104, 152902 CrossRef PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A, 1976, 32, 751 CrossRef.
- S. Ye, E. Song, E. Ma, S. Zhang, J. Wang, X. Chen, Q. Zhang and J. Qiu, Opt. Mater. Express, 2014, 4, 638 CrossRef.
- E. Maurice, G. Monnom, B. Dussardier, A. Saïssy, D. Ostrowsky and G. Baxter, Appl. Opt., 1995, 34, 8019 CrossRef CAS PubMed.
- Y. Zhao, C. Riemersma, F. Pietra, R. Koole, C. de Mello Donegá and A. Meijerink, ACS Nano, 2012, 6, 9058 CrossRef CAS PubMed.
- Z. Xia, X. Wang, Y. Wang, L. Liao and X. Jing, Inorg. Chem., 2011, 50, 10134 CrossRef CAS PubMed.
- J. Orive, J. L. Mesa, R. Balda, J. N. Fernández, J. S. Rodríguez Fernández, T. F. Rojo and M. A. I. Arriortua, Inorg. Chem., 2011, 50, 12463 CrossRef CAS PubMed.
- F. Liu, Y. Liang and Z. Pan, Phys. Rev. Lett., 2014, 113, 177401 CrossRef.
- K. Van den Eeckhout, P. F. Smet and D. Poelman, Materials, 2010, 3, 2536 CrossRef CAS PubMed.
- T. Suehiro, N. Hirosaki, R. J. Xie and T. Sato, Appl. Phys. Lett., 2009, 95, 051903 CrossRef PubMed.
- S. Zhang, Y. Huang, Y. Nakai, T. Tsuboi and H. J. Seo, J. Am. Ceram. Soc., 2011, 94, 2987 CrossRef CAS PubMed.
- Y. Ma, F. Xiao, S. Ye and Q. Zhang, J. Electrochem. Soc., 2012, 159, H358 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09095d |
| This journal is © The Royal Society of Chemistry 2015 |