Photosensitisation studies of silicone polymer doped with methylene blue and nanogold for antimicrobial applications†
M. J. Bovis*a,
S. Noimarkb,
J. H. Woodhamsa,
C. W. M. Kayc,
J. Weinerd,
W. J. Pevelerb,
A. Correiae,
M. Wilsone,
E. Allane,
I. P. Parkinb and
A. J. MacRoberta
aDivision of Surgery & Interventional Science, University College London, London, W1W 7EJ, UK. E-mail: m.bovis@ucl.ac.uk; Tel: +44 (0)207679 9069
bMaterials Chemistry Research Centre, Department of Chemistry, University College London, London, WC1H 0AJ, UK
cInstitute of Structural & Molecular Biology and London Centre of Nanotechnology, University College London, London, WC1E 6BT, UK
dDepartment of Chemistry, Imperial College London, London, SW7 2AZ, UK
eDivision of Microbial Diseases, UCL Eastman Dental Institute, University College London, London, WC1X 8LD, UK
First published on 12th June 2015
Abstract
Photosensitisation of polymers has important potential clinical applications such as the prevention of catheter-associated urinary tract infections (CAUTIs). Polymers incorporated with methylene blue (MB) and 2 nm gold nanoparticles (AuNPs) are effective in killing bacteria at the surface following low power visible illumination. Studies of medical-grade silicone polymer samples including segments from urinary catheters were carried out to investigate the generation of reactive oxygen species and the involvement of Type 1 and 2 mechanisms. Singlet oxygen was observed using direct phosphorescence detection and hydroxyl radical generation using electron paramagnetic resonance (EPR) spectroscopy; we conclude that both Type 1 and 2 mechanisms can operate with polymeric photosensitisation. Transmission electron microscopy (TEM) directly demonstrated the incorporation of AuNPs at the surface of the silicone. Using silicone doped with MB AuNPs, a ≥3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 reduction in the number of viable Staphylococcus epidermidis bacteria was achieved when exposed to low power laser light; prior sterilisation with ethylene oxide (EO) had no influence on efficacy.
log10 reduction in the number of viable Staphylococcus epidermidis bacteria was achieved when exposed to low power laser light; prior sterilisation with ethylene oxide (EO) had no influence on efficacy.
1 Introduction
Recent figures from The British National Audit Office suggest an estimated 5000 deaths in the UK are attributed to nosocomial (hospital-acquired) infections.1 One of the most common nosocomial infections is urinary tract infections (UTIs); 80% of which are associated with urinary catheterisation.2 Increasing drug-resistance in pathogens, combined with an associated NHS expenditure of £1 billion, warrants the need for alternative combative methods to reduce infection rates.One of the most frequently used polymeric medical devices is the urinary catheter, with over 5 million inserted annually in the UK.3 Microorganisms associated with CAUTIs typically originate from the skin microbiota of the patient or healthcare personnel manipulating the catheter device, whereby bacteria migrate from the insertion site on the outer surface of the catheter or the catheter lumen. Despite efforts to continuously improve recommended guidelines for sterile techniques in clinical practice and the employment of catheter flushes, antibiotic and antiseptic devices, infection rates remain alarmingly high.4 Silver alloy catheters and antibiotic catheters (minocycline/rifampicin- or nitrofurazone-coated) have been developed to combat these issues and have demonstrated significant antimicrobial efficacy in patients undergoing short-term catheterisation in clinical trials.5 However, results on their impact on the overall risk of UTIs in patients with long-term indwelling catheter were inconclusive.6 Moreover, the use of antibiotic-coated catheters is likely to further exacerbate microbial drug resistance in prolonged patient hospitalisation.
For many years, there has been considerable interest in the use of non-toxic photosensitising agents, for antimicrobial applications.7 Incorporation of these light-activated antimicrobial agents (LAAAs) into the catheter polymer may offer a viable alternative to current techniques to prevent catheter-associated infections particularly in the urinary tract. In the presence of molecular oxygen (O2), light activation of the LAAA can generate cytotoxic reactive oxygen species (ROS) in a process known as photodynamic therapy (PDT). PDT can occur via two different photochemical pathways to produce hydroxyl radicals (˙OH) and superoxide anions (O2˙−) via electron transfer processes (Type 1) or singlet oxygen (1O2) via energy transfer (Type 2). Photo-generated ROS then induce bacterial kill through oxidative damage to sensitive subcellular sites.8
LAAAs such as photosensitive dyes, MB, toluidine blue O (TBO) and indocyanine green (ICG), can be incorporated into polymers commonly used in medical devices through a simple ‘swell–encapsulation–shrink’ method due to the well-documented swelling of some polymers in certain organic solvents.9 In urinary catheter applications, low power laser light can be administered to activate embedded LAAA via intraluminal irradiation with an optical fibre. These modified polymers have exhibited potent light-activated antimicrobial properties, effecting the lethal photosensitisation of Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Escherichia coli and Staphylococcus epidermidis.10 Furthermore, despite the lack of intrinsic bactericidal properties reported with gold, previous studies using polymers impregnated with MB gold nanoparticles (NPs) have demonstrated antimicrobial activity following light activation at 660 nm, relative to polymers impregnated with MB alone.2–5,10–12 In comparison to larger gold NPs of ca. 5 and 20 nm diameter, incorporation of 2 nm gold particles (AuNPs) has proved to be the most effective for enhancing antimicrobial activity following visible light exposure of MB impregnated polymer13 and have been used here. The applications of MB as a photosensitiser have also recently been reviewed.14
One reason why the use of photosensitising agents for bacterial disinfection has attracted so much interest is that it appears unlikely that pathogens can develop resistance to the indiscriminate photo-oxidative damage induced by ROS.7,15 Other groups are also investigating the antibacterial efficacy of polymers incorporating photosensitising agents but have suggested that the Type 2 mechanism is dominant,16 however, there is evidence for the involvement of Type 1 processes depending on the choice of photosensitiser.17
The main aim of this study was to investigate the fundamental photochemical mechanisms underpinning anti-microbial activity in medical-grade silicone polymers embedded with MB AuNPs in a bid to address the clinical need for infection prevention and provide an alternative approach to the ongoing problem of antibiotic resistance. This was achieved by using a combination of optical and electron paramagnetic resonance (EPR) spectroscopic techniques following ROS generation of MB AuNP-impregnated polymers and aqueous solutions exposed to the polymer. Photobleaching and oxygen consumption studies were also carried out at light doses relevant to bactericidal conditions. In order to maximise translation to clinical applications, medical-grade silicone polymer samples, including commercially available silicone urinary catheters, were used in this study.
2 Experimental
2.1 Materials and methods
2.2 Material characterisation: uptake of MB and AuNPs
2.3 Spectroscopic studies of MB triplet state and ROS generation
 | ||
| Fig. 1 Experimental setup used to investigate dependence of 1O2 phosphorescence on oxygen levels controlled with nitrogen flushing. | ||
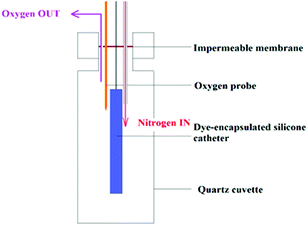
The time-resolved phosphorescence measurements were integrated by the multiscalar board (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 laser pulses over a 30 s collection time). Further measurements were recorded in which the sample was deoxygenated through the introduction of nitrogen gas (BOC Ltd, oxygen-free nitrogen) into the cuvette that was partially sealed using a Parafilm membrane (Fig. 1). A fibre-optic dissolved oxygen probe (Oxford Optronix Ltd., UK), was used to measure the partial pressure of O2 (mmHg) at selected intervals upon nitrogen flushing to deoxygenate the polymer which was carried out gradually over 1 h to enable re-equilibration.
000 laser pulses over a 30 s collection time). Further measurements were recorded in which the sample was deoxygenated through the introduction of nitrogen gas (BOC Ltd, oxygen-free nitrogen) into the cuvette that was partially sealed using a Parafilm membrane (Fig. 1). A fibre-optic dissolved oxygen probe (Oxford Optronix Ltd., UK), was used to measure the partial pressure of O2 (mmHg) at selected intervals upon nitrogen flushing to deoxygenate the polymer which was carried out gradually over 1 h to enable re-equilibration.
Fluorescence measurements were carried out using an LS50B Perkin-Elmer spectrofluorimeter equipped with a fibre-optic coupler (Perkin-Elmer, UK) controlled by FL Winlab software using excitation/emission wavelengths of 480/530 nm. MB AuNP-embedded silicone polymer flat sheets were cut into equal sized discs (5 mm diameter) using a hole-punch (Perforex 110) and placed in flat-bottomed wells in clear 96 well plates (TPP). 20 μL of 1 μM Sensor Green reagent (diluted in deuterated solvent, D2O) were deposited on to the surface of silicone polymer discs. Deuterated solutions were employed to enhance the 1O2 lifetime and thus increase probability of reaction with the fluorescence probe. Samples were exposed to 45 J cm−2 of red laser light at 670 nm (LD670C; Hamamatsu Photonics K.K.) using a microlens fibre (2 cm diameter spot) for 30 s (Fig. 2). Fluorescence readings were taken immediately before (0 pre-irradiation) and after (0 post-irradiation ≥ 30 min) irradiation of silicone samples. Fluorescence readings were acquired using a multimode bifurcated fibre-optic probe to provide front surface excitation/detection geometry. Control groups included silicone polymer samples without MB AuNPs or D2O alone (without Sensor Green).
2.4 Photobleaching and oxygen consumption measurements
2.5 Light activated studies
The samples in this experiment were tested against S. epidermidis, which is a frequent cause of CAUTIs. S. epidermidis RP62a was stored at −70 °C in Brain-Heart-Infusion broth (BHI, Oxoid) containing 20% (v/v) glycerol and propagated onto BHI agar (Oxoid) for a maximum of 2 sub-cultures at intervals of 2 weeks before returning to the frozen stock. BHI broth was inoculated with 1 bacterial colony and aerobically cultured for 24 h at 37 °C under static conditions. The bacterial pellet was washed and the suspension was serially diluted 1000-fold to obtain an inoculum of 106 colony forming units (CFU) mL−1. The inoculum in each experiment was confirmed by plating 10-fold serial dilutions onto Mannitol Salt Agar (MSA, Oxoid) for viable counts. Sterilised (EO) and unsterilised MB AuNP-encapsulated silicone polymer flat sheet swatches were divided into two groups and an inoculum volume of 25 μL of either bacterial suspension was deposited on each of the samples and covered with a sterile cover slip (22 mm × 22 mm). The samples were irradiated with 45 J cm−2 (155 mW, 15 min) of red laser light (Periowave, Ondine Biopharma Inc, Canada) at 660 nm using a microlens fibre (2 cm diameter illumination spot). These light treatment parameters were chosen based on optimal therapeutic outcomes in previously published studies with light-activated polymers containing MB AuNPs.10 A further set of samples (triplicate) was maintained under dark conditions at RT (15 min). Post-irradiation, the inoculated samples and cover slips were added to PBS (225 μL) and vortexed. The neat suspension and the 10-fold serial dilutions were plated on MSA for viable counts. The plates were incubated aerobically at 37 °C for 24 h. Each experiment contained two technical replicates and viable colonies for each strain were counted. The detection limit was 400 CFU mL−1.
3 Results
3.1 Characterisation of MB AuNP incorporation
A simple ‘swell–encapsulation–shrink’ method12 was used to incorporate the AuNPs into commercially available silicone catheters and silicone polymer flat sheets.TEM images of the AuNPs in solution showed the particles to be spherical, mono-disperse and nano-crystalline (Fig. 3A and B). Analysis of over 1000 particles gave an average size of 2.2 nm with a fairly narrow size distribution SD = 1.1 nm (Fig. 3C). In high resolution images (HR-TEM) of the NPs, lattice spacings of ∼0.235 nm were observed, correlating with the (111) plane of fcc gold (Fig. 3B). We confirmed the presence of AuNPs near the surface of 5 micron sections of MB AuNP-encapsulated samples where the same crystalline nanostructures, approximately 2 nm in diameter along the silicone polymer edge (white arrows), were observed (Fig. 3D).
Uptake efficiency studies of MB into silicone were carried out using a combination of absorption and fluorescence measurements. Fluorescence imaging enables more detailed spatial measurement of MB within thin sections of the polymer. The silicone samples were swelled in solutions comprising of acetone concentrations ranging from 0% through to 100% acetone and it was found that the presence of any substantial aqueous component in the swelling solution significantly hindered the polymer swelling and thus, the encapsulation of the MB (data not shown). By comparing different swelling regimes, the identification of optimal swelling conditions for the encapsulation of the MB was achieved. A 95% acetone solution proved to be the most effective. The effect of the immersion frequency (number of dips): immersion duration (approximate swelling time) on MB uptake and distribution in silicone urinary catheters was investigated using low power fluorescence microscopy.
A positive correlation was established between the two parameters, whereby the longer the catheter was exposed to the swelling solution (>30 min) and the higher the number of dips, the greater the uptake and penetration of MB into catheters (Fig. 4). At short times, MB uptake is mainly confined to the surface but for longer times, the MB penetrates throughout the catheter thickness. This swelling regime was further examined in silicone polymers by substituting the aqueous component of the swelling solution for an AuNP suspension, as these NPs have previously exhibited superior anti-microbial activity in polymers than MB alone.10,12
The distribution and uniformity of MB was examined at a depth of 100 μm from the surface of the silicone sheet samples using a high resolution fibre-optic probe based confocal laser fluorescence microendoscope. Fig. 5 shows single frames of silicone sheets impregnated with or without MB AuNPs. The intense white signal shown in Fig. 5B is the fluorescence signal detected from the MB following excitation at 488 nm. No signal was observed in the control silicone sample (no MB AuNPs), Fig. 5A. MB distribution is not fully uniform at the measured depth presumably due to small inhomogeneities observed as grey speckling in the image (Fig. 5B).
3.2 Spectroscopic characterisation and ROS generation
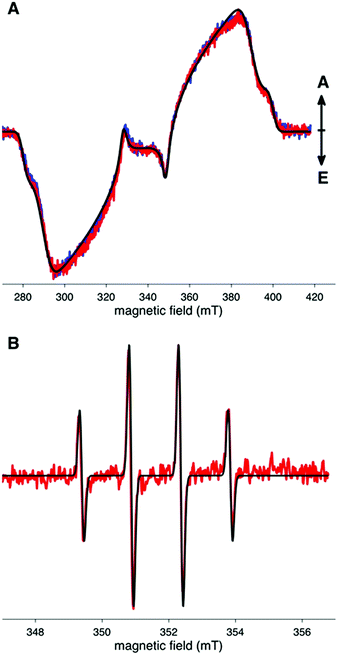
TR-EPR spectroscopy was used to detect photo-generated paramagnetic species present in the MB- and MB AuNP-embedded silicone catheter following laser excitation at 660 nm (Fig. 6A). Signals are typical of spin-polarized triplet states of aromatic molecules and are similar to previously observed TR-EPR spectra of MB in PVC catheters.12 We note that these spectra are essentially identical with or without AuNPs. Using the Easyspin19 toolbox running under a Matlab™ environment, the spectra were simulated using the following parameters: an isotropic g value equal to the free electron g value (gx![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gy
gy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gz = 2.00232); zero-field splitting parameters |D| = 1680 MHz and |E| = 370 MHz; and relative populations (px
gz = 2.00232); zero-field splitting parameters |D| = 1680 MHz and |E| = 370 MHz; and relative populations (px![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) py
py![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pz = 0
pz = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
CW-EPR in combination with the spin-trap DMPO was used to probe any radicals generated by the photo-excited triplet state dye (Fig. 6B). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 spectrum observed, with hyperfine coupling constants: α(1H) = 14.9 G, α(14N) = 14.9 G, is consistent with spin-trapped ˙OH. Identical spectra were observed with MB- or MB AuNP-encapsulated silicone catheters, suggesting that the presence of AuNPs does not affect the nature of the radicals formed.
1 spectrum observed, with hyperfine coupling constants: α(1H) = 14.9 G, α(14N) = 14.9 G, is consistent with spin-trapped ˙OH. Identical spectra were observed with MB- or MB AuNP-encapsulated silicone catheters, suggesting that the presence of AuNPs does not affect the nature of the radicals formed.
CW-EPR 2D-field delay measurements of the illuminated DMPO-catheter systems were also recorded, to establish whether the radical distribution evolved over time. No change in the radical distribution present in DMPO exposed to either MB- or MB AuNP-encapsulated silicone was detected over a period of 45 minutes (data not shown).
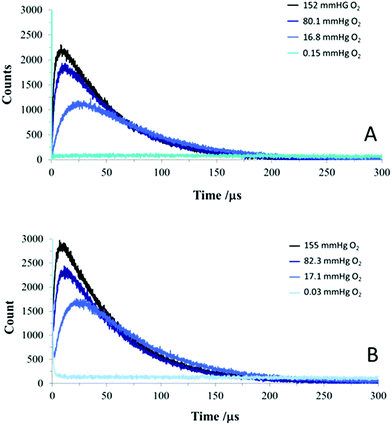
Fig. 7 shows time-resolved NIR signals recorded from the polymer samples irradiated using a 532 nm pulsed laser as a function of ambient oxygen partial pressure. At the lowest oxygen pressure (0.03 mmHg) no signal was detected (apart from a transient initial spike, due to residual MB fluorescence), therefore we can confidently assign the signals at higher oxygen levels to 1O2. Control samples without MB showed no signal. A steep rise in 1O2 production was observed immediately after the 3 ns laser pulse with the MB or MB AuNP-encapsulated silicone, followed by a slower decay. As more nitrogen gas was introduced into the system and the partial pressure of oxygen decreased, the rise time became longer for both polymers. These rise and fall kinetics are characteristic of the standard model of photosensitised 1O2 production via triplet state oxygen quenching using pulsed excitation (eqn (1)) with the 1O2 kinetics versus time (t) determined by the lifetime of the photosensitiser triplet state lifetime (τT) and 1O2 lifetime (τΔ) following initial excitation of the photosensitiser to the triplet state (3sens*) by the laser pulse.20 The triplet state can then be quenched rapidly by oxygen to yield 1O2, thus the triplet state lifetime exhibits an inverse Stern–Volmer dependence on the ground state oxygen concentration (eqn (2)).
To analyse the observed NIR kinetics, we employed a simple bi-exponential model and determined the rise and decay lifetimes and associated amplitudes, shown in Table 1. It is important to note that eqn (1) is symmetrical in τT and τΔ and the rise time can result either from the triplet state or 1O2 kinetics.
 | (1) |
 is the rate constant of energy transfer between the sensitiser triplet state and molecular oxygen, kTd is the rate constant for oxygen independent decays pathways,
is the rate constant of energy transfer between the sensitiser triplet state and molecular oxygen, kTd is the rate constant for oxygen independent decays pathways,  is the rate constant for quenching of the triplet state.
is the rate constant for quenching of the triplet state.
| PO2 (mmHg) | A1 | τ1 (μs) | A2 | τ2 (μs) |
|---|---|---|---|---|
| MB-encapsulated silicone catheter | ||||
| 16.8 | 1.98 | 76.4 | 1.79 | 12.1 |
| 43.5 | 2.98 | 60.4 | 1.70 | 7.0 |
| 80.1 | 2.56 | 60.2 | 1.41 | 5.2 |
| 152 | 2.87 | 59.3 | 1.19 | 3.8 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| MB AuNP-encapsulated silicone catheter | ||||
| 17.1 | 2.89 | 66.0 | 2.14 | 12.7 |
| 48.1 | 3.09 | 59.8 | 1.84 | 7.1 |
| 82.3 | 3.12 | 56.6 | 1.51 | 5.6 |
| 155 | 3.60 | 55.0 | 1.91 | 3.6 |
The triplet state lifetime is therefore determined as:
 | (2) |
Since the rise time component (τ2) shown in Table 1 is strongly dependent on the oxygen partial pressure with longer values observed at lower oxygen levels we assign this component to the triplet state lifetime of the MB, i.e. τ2 corresponds to τT in eqn (1). The reciprocal values of τ2 can be linearly fitted using Stern–Volmer analysis versus the oxygen pressure assuming that τ2 corresponds to the triplet state lifetime (Fig. S2†). Thus as the oxygen pressure is reduced the rate of triplet state quenching is reduced and correspondingly the rate of 1O2 photogeneration is also reduced which results in the slower rise in 1O2 production seen in the time-resolved traces (Fig. 7).
The decay in signal shown in Fig. 7 therefore corresponds to the 1O2 decay and the lifetimes (τ1) derived (Table 1) correspond to the 1O2 lifetime (τ2), giving a value of ca. 60 μs The bi-exponential model will be an approximation to the 1O2 kinetics given the heterogeneity of the polymer microenvironment, however, the relatively long 1O2 lifetime observed demonstrates that physicochemical quenching within the polymer matrix is much slower than in an aqueous environment where the τΔ is only ca. 3 μs.
The longer lifetime (τ1) corresponds to the decay lifetime of 1O2 at ca. 60 μs. Admittedly there is slight variation of the lifetime with oxygen pressure, but we attribute this to the simplicity of the bi-exponential model which works well in aqueous solution but in a heterogeneous polymer microenvironment the kinetics will inevitably be more complicated and difficult to model exactly.
The indirect detection of 1O2 released from the polymer into aqueous solution, prior to and following irradiation (IRR) of polymer samples, was measured using the Sensor Green (SG) reagent, diluted in D2O, for which the lifetime of 1O2 is approximately twenty times longer compared to H2O.21 The results shown in Fig. 8 demonstrate that the production of 1O2 is almost three-fold greater following irradiation of MB AuNP-encapsulated silicone polymer samples (MB AuNPs + SG) in comparison to control groups, which lacked either MB AuNPs in the catheter sample (w/o MB AuNPs + SG) or lacked the 1O2 detecting SG reagent (MB AuNPs + w/o SG). A small amount of sample autofluorescence was detected prior to light irradiation (time 0) for all three samples.
3.3 Photobleaching and oxygen consumption measurements
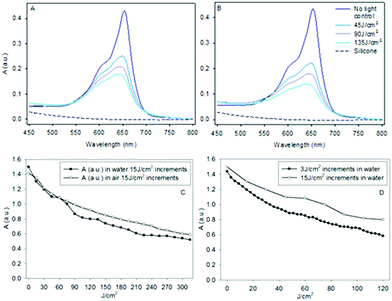
An absorbance spectrum of MB AuNP-encapsulated silicone sheets were taken before and after exposure to red laser light increments of 45 J cm−2 in air (Fig. 9A) and water (Fig. 9B), respectively. MB AuNP-encapsulated in silicone shows a characteristic MB monomer absorption peak at ∼650 nm and a lower intensity shoulder around ∼610 nm. The weak attenuation observed with the control silicone samples (w/o MB AuNP) is likely due to small light scattering losses. The absorption spectra of MB in the silicone polymer sheets and catheter samples gave similar peak absorption wavelengths of 652 nm and 651 nm, respectively (Fig. S1†) within experimental error, which is in agreement with a previous study on silicone polymers.13This MB peak (∼652 nm) is blue-shifted compared to that observed in aqueous solution (∼665 nm), which is attributed to the different microenvironments (e.g. dielectric constant). Absorption spectra of monomeric and dimeric MB have been documented previously by Baptista and colleagues22 who showed that the dimer exhibits peak absorption at ∼590 nm, which is significantly shorter than that observed here, suggesting the presence of predominantly monomeric species. The concentration of MB in the samples is estimated to be ∼40 μM, assuming a similar extinction coefficient for monomeric MB as found for water.
After laser irradiation of the samples in air or water (since catheters are exposed to water under clinical conditions), bleaching of the MB absorption was observed (Fig. 9A and B). The change in absorbance at ∼650 nm was measured following incremental light doses of 15 J cm−2 over 5 min, delivering a total energy of 315 J cm−2 (21 illuminations), which resulted in a steady decline in absorbance, as shown in Fig. 9C. MB photobleaching appeared to be comparable with either short or long energy increments of laser light delivery (Fig. 9D) with minimal differences observed between air and water. However, it is noticeable that the decline in absorbance was not uniform versus wavelength across the MB spectra, as shown in Fig. 9A and B. The difference in absorbance between the first light treatment (45 J cm−2) and the total light dose (135 J cm−2) was two-fold lower at the smaller 590 nm shoulder than the decrease measured at the peak wavelength (∼652 nm) following deduction of control values (w/o MB).
The O2 available for PDT of MB AuNP-encapsulated in silicone is dependent on the oxygen diffusion gradient through the silicone matrix and the existing available oxygen already present in the silicone. Silicone is porous to O2 and by inserting a fibre-optic oxygen needle sensor probe into each MB AuNP-encapsulated silicone sheet samples, the consumption of oxygen within the polymer could be measured during light delivery. Fig. 10 shows the changes in oxygen partial pressure (PO2) during and after two subsequent doses of light (670 nm, 45 J cm−2 at 15 mW cm−2) given to the same MB AuNP-encapsulated silicone samples. Initial PO2, prior to light, ranges from 155–175 mmHg, which is similar to PO2 in room air (approx. 160 mmHg), demonstrating the porosity of the silicone (Fig. 10A). During the first light delivery, within the initial 10 min, PO2 falls to its lowest point at a minimum of 20 mmHg, although this varied between samples and was most likely due to the positioning of the PO2 fibre-optic probe within the silicone sample. A steady recovery of oxygen was observed during the remaining light delivery period where within 20 min post-PDT PO2 was close to pre-PDT baseline levels, ranging from 130–150 mmHg. During the second light delivery dose, 45 J cm−2 at 15 mW cm−2 (Fig. 10B), there was little change in PO2 and this remained constant up to 20 min post-PDT.
3.4 Effect of sterilisation on antimicrobial activity
Sterilisation (EO) of MB AuNP-encapsulated silicone polymer flat sheets, prior to microbial testing, did not impact on the demonstrated light-activated antimicrobial activity (∼660 nm irradiation) compared to unsterilised samples (Fig. 11). MB AuNP-embedded silicone samples inoculated with S. epidermidis demonstrated a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 reduction in viable colony counts (CFU mL−1), scarcely above the detection limit, when exposed to low power laser light at 660 nm for 15 min (155 mW). Prior EO sterilisation did not decrease the demonstrated light-activated antimicrobial properties, impact the MB concentration (absorption remains constant) or inhibit 1O2 generation (data not shown). We have shown previously10 in control studies using medical grade silicone samples that a reduction in CFU is only observed when MB and light are used together. Incorporation of AuNPs alone with or without light induces no measurable antibacterial activity.
log10 reduction in viable colony counts (CFU mL−1), scarcely above the detection limit, when exposed to low power laser light at 660 nm for 15 min (155 mW). Prior EO sterilisation did not decrease the demonstrated light-activated antimicrobial properties, impact the MB concentration (absorption remains constant) or inhibit 1O2 generation (data not shown). We have shown previously10 in control studies using medical grade silicone samples that a reduction in CFU is only observed when MB and light are used together. Incorporation of AuNPs alone with or without light induces no measurable antibacterial activity.
4 Discussion
In these studies, the photophysical properties of medical-grade silicone polymer impregnated with a non-toxic photosensitive dye (MB) and AuNPs were determined using a range of spectroscopic techniques. The aim was to characterise the properties of silicone polymers using relevant bactericidal light doses to guide the development of novel catheter devices for clinical applications, in particular, prevention of catheter-associated infections in the urinary tract. In the presence of O2, photoactivation of MB results in ROS generation leading to bactericidal activity. The additional incorporation of MB with AuNPs of 2 nm diameter into polymeric substrates has been previously shown to enhance the antimicrobial properties in polymers over MB alone,10 despite being too small for the plasmon-resonance effect to take place. It is assumed that MB can interact with the AuNPs via surface adsorption or dynamically via local diffusion within the matrix, which in turn could influence the photoactivity of the photosensitised polymer, as discussed below.A previously established simple ‘swell–encapsulation–shrink’10,12 method was used to incorporate MB AuNPs into commercially available silicone urinary catheters and medical-grade silicone flat sheets. In these previous studies, imaging of AuNPs uptake within the surface was not performed. In this study, the uptake of the AuNPs within the polymer surface was directly observed through TEM (Fig. 3), which to our knowledge, has not previously been demonstrated. Overall TEM images displayed monodisperse, spherical AuNPs with an average size of 2.2 nm (SD = 1.1 nm), concentrated at the polymer surface (Fig. 3D).
Corresponding uptake of conjugated photoactive dye (MB) was assessed through fluorescence microscopy. Fluorescence images of MB dyed urinary catheter shaft sections confirmed the presence of MB throughout the samples (Fig. 4). Since MB dimers are non-fluorescent, only monomeric MB fluorescence is imaged. The fluorescence intensity increased with the immersion frequency (number of dips) and duration (2 ≥ 34 min) in a 95% acetone swelling solution. The more uniform distribution contracts with a previous study on crystal violet which was concentrated near the surface of the catheter sections.23 There was no detectable difference between the absorbance spectra of MB and MB AuNP-encapsulated silicone catheter sections prepared using identical ‘swell–encapsulation–shrink’ methods for 24 h (Fig. S1†). However, these are bulk measurements based on the full sample thickness and local changes in absorbance within a shallow layer near the surface owing to the presence of the AuNPs would be difficult to discern from these measurements.
TR-EPR spectra, obtained at 300 K, demonstrated that the presence of AuNPs does not effect a change in the TR-EPR signals in terms of either the zero field splitting or relative populations (Fig. 6). This is in contrast to a previous TR-EPR investigation of PVC-encapsulated MB at 90 K in which a small enhancement in triplet state signal intensity was noted when AuNPs were present,12 although the study used a six-fold higher concentration of AuNPs in the swelling solution.
CW-EPR in combination with a spin-trap was used to probe the radicals subsequently generated by the photo-excited triplet state dye. CW-EPR spectra of DMPO solutions exposed to illuminated MB- or MB AuNP-encapsulated silicone (Fig. 6) show the presence of the DMPO-OH spin-adduct. OH˙ can be generated via metal-catalysed decomposition of hydrogen peroxide (H2O2) via the Fenton reaction, where H2O2 is generated from dismutation of O2˙−. We saw no evidence for the formation of superoxide derived radicals in the EPR spectrum, however, this does not rule out their presence, since such adducts are relatively short-lived. The superoxide dismutation reaction will also reduce the steady-state superoxide levels. Although superoxide dismutation is very slow in aprotic solvents this process could also take place within the polymer surface, which will be partly hydrated, and could further diminish O2˙− levels in the surrounding aqueous solution containing the spin-trapping agent. It is also possible that the dismutation rate can be enhanced through interaction with the NP surface. We did attempt to detect superoxide release from the polymer into solution using the ferricytochrome C assay24 but no evidence was found for ferricytochrome reduction by superoxide (data not shown).
Baptista and colleagues22 have summarised the pathways for ROS generation from MB photoexcitation. Under aerated conditions, photoexcited MB in its triplet state will undergo quenching by oxygen to generate 1O2 via the Type 2 route. But triplet MB can also generate MB˙ radicals via a Type 1 electron transfer mechanism with ground-state MB, and the MB˙ radicals can then react with O2 to form O2˙−. However, the radical product, MB˙, generated from ground-state and triplet state interaction is short-lived, which will reduce its steady-state levels owing to quenching by oxygen to form O2˙−. MB˙ dimers may also contribute to superoxide generation via a Type 1 process.22 Since the MB concentration in the polymer reaches ca. 40 μM, which is relatively high for photosensitisation studies and probably even higher near the surface, it is reasonable to assume that these Type 1 mechanisms can operate.
In CW-EPR studies, no carbon-centred radicals were detected, such as MB˙, which could suggest firstly, that MB is stable with respect to leaching from the polymer and secondly, that limited interactions occur between photo-excited MB molecules and close proximity ground state MB molecules. However, as discussed above, the steady-state levels of the MB˙ radicals are likely to be low owing to rapid electron-transfer to O2. However, it is possible that the Type 1 process could be enhanced in the presence of AuNPs. Electron-transfer between AuNPs (similar diameter to those used herein) and porphyrin–fullerene dyads has been shown to occur under visible light excitation.25 It is also possible that the polymer can also act as an electron donor to the MB triplet state to form MB radicals. Such a mechanism is analogous to that proposed for xanthene photosensitising dyes incorporated within a polyvinyl alcohol matrix which results in photoreduction and bleaching of the dye to the colourless leuco form.26
The involvement of the Type 2 process was also confirmed through direct NIR detection of 1O2 in the polymer. MB AuNP-encapsulated silicone catheters were excited at 532 nm and the production of 1O2 was measured through the time-resolved detection of NIR 1O2 phosphorescence, emitted at 1270 nm. Flushing the sample chamber with nitrogen was used to gradually deoxygenate the samples, in order to examine the effects of decreasing the partial pressure of oxygen (PO2) on the formation of 1O2. As more nitrogen gas was introduced into the system and the PO2 decreased, the 1O2 rise time became longer (Fig. 7), whereas the decay lifetimes were similar. The orthogonal geometry used for NIR-1O2 phosphorescence detection is highly sensitive to the placement of the samples and we did not attempt to ascertain whether the MB- or the MB AuNP-encapsulated silicone catheters could generate a greater yield of 1O2.
Analysis of the data (Fig. 7 and Table 1) demonstrates that the 1O2 signal can be separated into two main components with lifetimes τ1 and τ2, where τ1 corresponds to physicochemical quenching of 1O2 with other species such as the polymer matrix, whereas the latter component with a shorter lifetime (τ2) correlates to the 1O2 photogeneration lifetime, which is equivalent to the decay lifetime of the MB triplet state. The key assumption here is that the dominant deactivation pathway of the MB triplet state under aerated conditions is quenching by oxygen, a fraction of which will generate 1O2. The reduction in τ2 with lower oxygen levels is consistent with the expected strong quenching of the MB triplet state monomer by O2, and shows good correlation with Stern–Volmer analysis (Fig. S2†). The oxygen dependence rules out a significant contribution from photoexcited MB dimers, which are too short-lived.22 Quenching of the triplet state by the polymer matrix is also present, probably due to physical processes, as indicated by the Stern–Volmer analysis. The 1O2 lifetime was measured to be ca. 60 μs, which is significantly longer than observed in water (ca. 3 μs) and shows that the silicone matrix does not deactivate 1O2 significantly.
It is important to note, however, that 1O2 has a limited diffusion distance even with a lifetime of ca. 60 μs. For example the average distance travelled by 1O2 in D2O over 65 μs is approximately 0.62 μm.27 For polysiloxane polymers using a value for the diffusion coefficient D = 10−6 cm2 s−1 at 300 K the average diffusion distance is <0.1 μm [root-mean-square linear displacement] ∼ [(2τΔD)1/2].28 The fact that the AuNPs are presumed to be located close to the surface may be significant, assuming there is direct interaction between the NPs and the photosensitiser. Once released into aqueous solution the 1O2 lifetime is reduced considerably through physical quenching by H2O, which in turn limits its diffusion distance. Nevertheless, we were able to demonstrate indirect detection of 1O2 release from the polymer into surrounding solution using a hydrophilic chemical trapping reagent (Sensor Green) which emits a green fluorescence after oxidation by 1O2 (Fig. 8). Deuterated water (D2O) significantly increases the lifetime of 1O2 and was therefore used to monitor 1O2 production of catheter samples in real time. Similar results were found under identical conditions when using PBS but the fluorescence detected was much less due to the reduced lifetime of 1O2 in solutions containing H2O (Fig. S4†).
Overall, the experiments using both direct and indirect detection techniques demonstrate that the MB- and MB AuNP-encapsulated silicone polymer can undergo a Type 2 photochemical pathway upon excitation at 532 nm, generating the transient 1O2 species. Moreover, deoxygenation of the sample systems using nitrogen gas indicates that a severe depletion in the generation of 1O2 occurs only at conditions significantly more anaerobic than those typically found in a physiological setting. Consequently, for clinical applications, the light-activated antimicrobial activity via a Type 2 photochemical pathway, should not be limited as a result of the insufficient availability of O2.
It is imperative that the MB AuNP-encapsulated silicone catheters do not exhibit significant MB photodegradation (or photobleaching) under light treatment regimes, since patients undergoing urinary catheterisation may require an indwelling catheter device for prolonged periods, and several cycles of laser exposure. Photobleaching of dyes in different polymer matrices has been reported,29 and MB photobleaching generally results in the formation of a colourless ‘leuco’ form most likely via Type 1 photoreduction mechanisms, either involving electron transfer from MB or other substrates to the MB triplet state.30 Photobleaching of photosensitisers is known to be a limiting factor in PDT since the efficiency of ROS generation will decline during irradiation unless a photoactive degradation product is also generated.31 In the MB AuNP-encapsulated silicone polymer for a laser light dose of 45 J cm−2 (the same light dose as used bactericidal studies, Fig. 11) MB bleaching at the peak absorption was limited to 25% (Fig. 9), therefore was not a significant limiting factor in the anti-microbial studies. Moreover, when the samples were placed in water, instead of air, no significant change in photobleaching rate was observed, irrespective of variation in light cycle delivery, relevant to their potential clinical use. We did, however, observe a significantly lower rate of bleaching at 590 nm that may be due to the presence of underlying dimer absorption. This assumes that the dimer is more resistant to photobleaching using the light source employed, which was matched to the peak (monomeric) MB absorption, where dimer absorption is much weaker.22 Alternatively, it is possible that absorption from a photoproduct of the monomer bleaching results in the slower apparent rate of bleaching. The presence of dimers is likely to be relevant to the mechanism of bacterial killing where broad-band white light is used, as discussed below. Longer term photobleaching studies (Fig. S3†) suggest some recovery of MB absorption can take place after 1 week, presumably via oxidation of the photoreduced leuco form. This may be relevant to long-term indwelling catheter usage.
Overall, the limited photobleaching of the MB AuNP-encapsulated silicone catheter sections and limited recovery after storage in dark conditions is encouraging but a balance must be achieved between MB AuNP-incorporation and light transmission through the catheter for optimal treatment. Lower concentrations of MB could be used to render improved light transmission, although this may in turn adversely affect antimicrobial efficacy of the device as higher light doses may be required to induce the same degree of cell kill, affecting photobleaching rates.
The observations on oxygen consumption within the polymer during laser illumination shown in Fig. 10 are also consistent with the presence of Type 1 and 2 mechanisms. The rate of oxygen consumption was highest during the first light dose. 1O2 may initially be scavenged by residual (unsaturated) monomers in the silicone matrix, and this chemical trapping of 1O2 in the polymer leads to a reduced amount of O2 present in the polymer before replenishment by oxygen diffusing into the matrix. 1O2 may also react with MB which would also result in oxygen depletion but since MB photodegradation occurs in parallel this process will become less important with time. Since the rate of bleaching was much slower during subsequent illumination this suggests the presence of a ‘passivation’ effect consistent with scavenging of oxidisable substrates within the polymer. Surface passivation of polymer films has been reported through photooxygenation reactions in support of this conclusion.32 Since deoxygenation can be counteracted by a slower rate of light delivery to enable more oxygen back-diffusion, our results would suggest that a fractionated illumination regimen could improve efficacy as attested by in vivo PDT studies.33–35
However, from the experimental data obtained thus far, it is difficult to ascertain which of these photochemical pathways is predominant. Our spectroscopic studies are also based on bulk measurements rather than the surface, in which case the diffusion distance of the ROS involved must also be considered and, as discussed above, this will be a limiting factor with 1O2. Interaction of MB with AuNPs has been reported to enhance MB absorption which will favour both Type 1 and 2 processes36,37 but this will only occur near the surface where the NPs are located and may not therefore be apparent from our bulk phase optical absorption studies. Likewise MB interaction with AuNPs may promote a Type 1 electron transfer process. Such a local enhancement will result in a steeper concentration gradient of photogenerated ROS very close to the surface, although this will be rapidly diluted away from the surface in surrounding medium.
As discussed above, the drawback with the Type 2 mechanism is the limited diffusion distance of 1O2 in water (and biofilms) which must reduce the efficiency of bacterial deactivation. This could be mitigated by the formation of longer-lived hydroperoxides which could diffuse within the biofilm or generate other radicals such as peroxyl.38 The observation of highly reactive OH˙ using EPR spectroscopy, which we presume are formed from H2O2, leads us to speculate that H2O2 generated via the Type 1 mechanism may also contribute to bacterial kill. It has previously been shown that H2O2 is capable of mediating damage to photosensitised cell monolayers outside the illumination zone owing to its stability and longer diffusion distance.39,40 Hydroperoxides may also be involved since these may be formed via hydrogen abstraction reactions involving hydroxyl radicals.38
The relative efficacy of Type 1 and 2 mechanisms will depend on the pathogen to be targeted since intracellular scavengers can mitigate the efficacy of the ROS, and the photosensitiser employed will also have to be taken into account. Further studies should investigate the use of ROS inhibitors, although as Hamblin and colleagues have shown, care has to be exercised in the interpretation of the data.41
Testing of the antimicrobial activity with S. epidermidis demonstrated a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 reduction in the number of bacteria when MB AuNP-embedded silicone polymer samples were exposed to low power laser light at 660 nm giving a total fluence of 45 J cm−2 compared to control groups that received no light treatment, as shown in Fig. 11. Similar results have been documented previously with MB AuNPs in PVC polymer samples.10,12 However, the key conclusion from the data shown in Fig. 11 is that sterilisation (EO) of MB AuNP samples prior to microbiological testing did not reduce their photobactericidal activity. This is relevant to future clinical testing of such catheters since they would have to be sterilised using this standard clinically-approved method prior to use.
log10 reduction in the number of bacteria when MB AuNP-embedded silicone polymer samples were exposed to low power laser light at 660 nm giving a total fluence of 45 J cm−2 compared to control groups that received no light treatment, as shown in Fig. 11. Similar results have been documented previously with MB AuNPs in PVC polymer samples.10,12 However, the key conclusion from the data shown in Fig. 11 is that sterilisation (EO) of MB AuNP samples prior to microbiological testing did not reduce their photobactericidal activity. This is relevant to future clinical testing of such catheters since they would have to be sterilised using this standard clinically-approved method prior to use.
5 Conclusions
We have demonstrated incorporation of MB AuNPs into medical-grade silicone polymer, which provides an effective means of antimicrobial treatment following light exposure. Our studies have indicated involvement of both Type 1 and 2 mechanisms for cytotoxic ROS generation. In existing PDT applications, more emphasis has been placed on the involvement of the Type 2 mechanism. However, for surface photoinactivation and release of ROS from the photosensitised polymer surface, the Type 1 mechanisms warrants further attention. This is due the limited diffusion distance of 1O2 and the requirement of sufficient external ROS generation to exert a lethal effect upon proximal bacteria. Photobleaching and oxygen consumption studies demonstrated that these factors are not critical in determining anti-bactericidal efficacy. Furthermore, the antimicrobial effect is not modified prior to sterilisation (EO) of polymers. In summary, our results lend support to the use of this technique for preventative treatment against CAUTIs and amelioration of the associated healthcare expenditure and morbidity.Acknowledgements
This work was supported by the Medical Research Council. Sacha Noimark is grateful for support from Ondine Biomedical Inc. We also acknowledge assistance with the confocal laser studies from Dr Baptiste Allain and assistance with laser equipment from Sandy Mosse.References
- J. Bourn, The Management and Control of Hospital Acquired Infections in Acute NHS Trusts in England (1999-00), 2000 Search PubMed.
- C. S. Bryan and K. L. Reynolds, J. Urol., 1984, 132, 494–498 CAS.
- S. Noimark, C. W. Dunnill, M. Wilson and I. P. Parkin, Chem. Soc. Rev., 2009, 38, 3435–3448 RSC.
- D. G. Maki and P. A. Tambyah, Emerging Infect. Dis., 2001, 7, 342–347 CrossRef CAS PubMed.
- K. Schumm and T. B. Lam, Cochrane Database Syst. Rev., 2008, CD004013 CAS.
- P. Jahn, M. Preuss, A. Kernig, A. Seifert-Huhmer and G. Langer, Cochrane Database Syst. Rev., 2007, CD004997 CAS.
- M. Wainwright, J. Antimicrob. Chemother., 2012, 67, 787–788 CrossRef CAS PubMed.
- M. Gracanin, C. L. Hawkins, D. I. Pattison and M. J. Davies, Free Radical Biol. Med., 2009, 47, 92–102 CrossRef CAS PubMed.
- J. N. Lee, C. Park and G. M. Whitesides, Anal. Chem., 2003, 75, 6544–6554 CrossRef CAS PubMed.
- S. Perni, C. Piccirillo, J. Pratten, P. Prokopovich, W. Chrzanowski, I. P. Parkin and M. Wilson, Biomaterials, 2009, 30, 89–93 CrossRef CAS PubMed.
- S. Perni, P. Prokopovich, J. Pratten, I. P. Parkin and M. Wilson, Photochem. Photobiol. Sci., 2011, 10, 712–720 CAS.
- S. Noimark, C. W. Dunnill, C. W. M. Kay, S. Perni, P. Prokopovich, S. Ismail, M. Wilson and I. P. Parkin, J. Mater. Chem., 2012, 22, 15388–15396 RSC.
- S. Perni, C. Piccirillo, A. Kafizas, M. Uppal, J. Pratten, M. Wilson and I. P. Parkin, J. Cluster Sci., 2010, 21, 427–438 CrossRef CAS PubMed.
- J. P. Tardivo, A. Del Giglio, C. S. de Oliveira, D. S. Gabrielli, H. C. Junqueira, D. B. Tada, D. Severino, R. D. F. Turchiello and M. S. Baptista, Photodiagn. Photodyn. Ther., 2005, 2, 175–191 CrossRef CAS.
- F. Vatansever, W. C. M. A. de Melo, P. Avci, D. Vecchio, M. Sadasivam, A. Gupta, R. Chandran, M. Karimi, N. A. Parizotto, R. Yin, G. P. Tegos and M. R. Hamblin, FEMS Microbiol. Rev., 2013, 37, 955–989 CAS.
- A. Felgentrager, T. Maisch, A. Spath, J. A. Schroder and W. Baumler, Phys. Chem. Chem. Phys., 2014, 16, 20598–20607 RSC.
- P. Mroz, G. P. Tegos, H. Gali, T. Wharton, T. Sarna and M. R. Hamblin, Photochem. Photobiol. Sci., 2007, 6, 1139–1149 CAS.
- C. Flors, M. J. Fryer, J. Waring, B. Reeder, U. Bechtold, P. M. Mullineaux, S. Nonell, M. T. Wilson and N. R. Baker, J. Exp. Bot., 2006, 57, 1725–1734 CrossRef CAS PubMed.
- S. Stoll and A. Schweiger, J. Magn. Reson., 2006, 178, 42–55 CrossRef CAS PubMed.
- A. Jimenez-Banzo, X. Ragas, P. Kapusta and S. Nonell, Photochem. Photobiol. Sci., 2008, 7, 1003–1010 CAS.
- S. Nonell, M. Garcia-Diaz, J. L. Viladot and R. Delgado, Int. J. Cosmet. Sci., 2013, 35, 272–280 CrossRef CAS PubMed.
- H. C. Junqueira, D. Severino, L. G. Dias, M. S. Gugliotti and M. S. Baptista, Phys. Chem. Chem. Phys., 2002, 4, 2320–2328 RSC.
- S. Noimark, M. Bovis, A. J. MacRobert, A. Correia, E. Allan, M. Wilson and I. P. Parkin, RSC Adv., 2013, 3, 18383–18394 RSC.
- Z. X. Zhou, Q. Q. Li, W. Liu, L. N. Zhang, X. L. Wang, H. T. Ma, W. Sheng, Z. L. Li, Y. Zeng and R. G. Zhong, J. Photochem. Photobiol., B, 2012, 117, 47–54 CrossRef CAS PubMed.
- A. Kotiaho, R. M. Lahtinen, N. V. Tkachenko, A. Efimov, A. Kira, H. Imahori and H. Lemmetyinen, Langmuir, 2007, 23, 13117–13125 CrossRef CAS PubMed.
- M. Talhavini, W. Corradini and T. D. Z. Atvars, J. Photochem. Photobiol., A, 2001, 139, 187–197 CrossRef CAS.
- I. Zebger, L. Poulsen, Z. Gao, L. K. Andersen and P. R. Ogilby, Langmuir, 2003, 19, 8927–8933 CrossRef CAS.
- S. G. Charati and S. A. Stern, Macromolecules, 1998, 31, 5529–5535 CrossRef CAS.
- B. Enko, S. M. Borisov, J. Regensburger, W. Baumler, G. Gescheidt and I. Klimant, J. Phys. Chem. A, 2013, 117, 8873–8882 CrossRef CAS PubMed.
- J. Chen, T. C. Cesario and P. M. Rentzepis, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 33–38 CrossRef CAS PubMed.
- L. Kunz and A. J. MacRobert, Photochem. Photobiol., 2002, 75, 28–35 CrossRef CAS.
- E. S. Goncalves and P. R. Ogilby, Langmuir, 2008, 24, 9056–9065 CrossRef CAS PubMed.
- A. Curnow, B. W. McIlroy, M. J. Postle-Hacon, A. J. MacRobert and S. G. Bown, Photochem. Photobiol., 1999, 69, 71–76 CrossRef CAS PubMed.
- A. Curnow, J. C. Haller and S. G. Bown, J. Photochem. Photobiol., B, 2000, 58, 149–155 CrossRef CAS.
- J. H. Woodhams, A. J. MacRobert and S. G. Bown, Photochem. Photobiol. Sci., 2007, 6, 1246–1256 CAS.
- N. Narband, M. Uppal, C. W. Dunnill, G. Hyett, M. Wilson and I. P. Parkin, Phys. Chem. Chem. Phys., 2009, 11, 10513–10518 RSC.
- H. Kitching, A. J. Kenyon and I. P. Parkin, Phys. Chem. Chem. Phys., 2014, 16, 6050–6059 RSC.
- A. W. Girotti, Free Radical Biol. Med., 2008, 44, 956–968 CrossRef CAS PubMed.
- N. Rubio, S. P. Fleury and R. W. Redmond, Photochem. Photobiol. Sci., 2009, 8, 457–464 CAS.
- R. W. Redmond and I. E. Kochevar, Photochem. Photobiol., 2006, 82, 1178–1186 CrossRef CAS PubMed.
- L. Y. Huang, T. G. St Denis, Y. Xuan, Y. Y. Huang, M. Tanaka, A. Zadlo, T. Sarna and M. R. Hamblin, Free Radical Biol. Med., 2012, 53, 2062–2071 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09045h |
| This journal is © The Royal Society of Chemistry 2015 |