Enhanced properties of fiberglass-reinforced photocurable resin pile by introducing different fiberglass surface treatments and their biological evolution†
Mei Liua,
Jianshan Chena,
Xiaokun Hua,
Yueming Dua,
Yang Xiaa,
Ning Gub and
Feimin Zhang*ab
aJiangsu Key Laboratory of Oral Diseases, Nanjing Medical University, Nanjing 210029, China. E-mail: fmzhang@njmu.edu.cn
bSuzhou Institute & Collaborative Innovation Center of Suzhou Nano Science and Technology, Southeast University, Suzhou 215000, China
First published on 6th August 2015
Abstract
We aimed to investigate the influence of different fiberglass surface pretreatments on the interfacial and mechanical properties of fiberglass-reinforced photocurable (FRP) resin pile and evaluate their cytotoxicity. The fiberglass was treated by different types, such as heat and acid treatment, and then modified by KH570, which were immersed in a laboratory-prepared photocurable resin substrate to prepare FRP resin piles. Scanning electron microscope (SEM), thermogravimetric analyses (TGA) and Fourier transform infrared spectrum (FT-IR) were used to characterize the morphologies and structures. Furthermore, the flexural modulus, bending strength, and bending load of the FRP resin piles were analyzed and the cytotoxicity on L929 cells were measured via methyl thiazol tetrazolium (MTT) assay. The results showed that high treatment could enhance the combination of the fiberglass with KH570 and then mechanical properties of the FRP resin piles, but acid treatment could reduce the performance of the FRP resin piles. MTT assay revealed the low cytotoxicity, which could be a potential application.
Introduction
Post–core crown restorations are currently the most common method to treat big dentinal body defects. This method combines post, residual dentin, and crown restorations to restore a tooth. This method not only enhances the flexural capacity of residual dentin but it also provides support retainers for restorations. Previously, casting metal posts were widely used to restore teeth in the clinical setting. However, casting metal posts were associated with problems such as dental root fracture due to its high elastic modulus and these posts were also associated with considerable tooth preparation.1 Recent developments in materials science have resulted in the introduction of fiber-reinforced resin composite material (FRC) for use in dental procedures. FRC has many merits such as high strength, high modulus, easy formability, insulativity, corrosion resistance, and fatigue resistance.2,3 Consequently, it has a wide range of applications in many fields and its use as a dental material has been appreciated. Dental fiber post resin consists of parallel fiber bundle and resin matrix, which fuses these fiber bundles. The elasticity modulus of this material is similar to that of dentin.4–6 After bonding, dental fiber post and dental tissues become homogeneous.7 As a result, stress, which is responsible for tooth fracture, can be avoided when this material is used. Fiber post–core systems can reduce tooth preparation for the crown at a time, which decreases the number of visits and improves treatment efficiency consequently reducing the incidence of failure due to secondary infection of the root canal. Moreover, fiber post has been shown to have good biocompatibility. Furthermore, this material has many benefits such as avoiding the corrosion associated with metal post systems and it has an aesthetically desirable appearance. Furthermore, fiber post is more in line with the principles of aesthetic repair than traditional metal post–core systems.8,9 However, dental fiber post is still associated with common problems like fracture due to insufficient strength and falling off after cementing. Therefore, improving the mechanical strength of fiber post would be beneficial. FRC consists of two types of materials each with different properties: its mechanical property is determined by the mechanical strength of the fiber and resin, together with the strength of their bonding interface.10,11 Improving the strength of the bonding interface between the resin and fiber will improve the mechanical property of the FRC post,12–14 which can be achieved by pretreating the fiber surface. Surface treatment of fiberglass is a necessary and complex procedure. Experimental results show that heating processing is able to remove original rubber and decompose organic layer on surface of fiber, exposing radical groups. Then combination of fiber and silane coupling agent is enhanced. On the other hand, acid treatment increases hydroxide radicals on surface of fiberglass, improving its surface energy, augmenting percent grafting of silane coupling agent,15 further strengthening combination of fiber and resin, and finally mechanical property of FRC post is reinforced. However, the surface morphology of fiberglass is damaged by the simultaneous application of acid and heat,16 reducing the strength of the fiberglass and affecting the mechanical property of the FRC post.17 Therefore, in this study, we used a laboratory prepared photocurable resin matrix to fabricate an FRC post by heat treatment, acid etching, and silanization of the fiberglass surface. Then, we studied the interfacial property of fiberglass and resin and the effects of heat and acid etching on the mechanical property of FRC and verified the cytocompatibility of the fabricated post.Experimental section
Materials
High-strength fiberglass (diameter: 11 μm, Sinoma Technology Co., Ltd), resin matrix including Bisphenol A-glycidyl methacrylate (Bis-GMA, AR, Sigma-Aldrich, USA) and triethylene glycol dimethacrylate (TEGDMA; AR, Shanghai Titan Chemical Co., Ltd), photoinitiator system including camphorquinone (CQ, Shanghai Chemical Technology Co., Ltd) and dimethylaminoethyl methacrylate (DMAEMA; AR, Shanghai Titan Chemical Co., Ltd), HCl (AR, Liyang Dongfang Chemical Reagent Co., Ltd) and 3-(trimethoxysilyl)propyl methacrylate (KH570, AR, Sigma-Aldrich, USA) were used without purification. Commercial RTD piles were purchased from France RTD Company.Surface treatment of fiberglass
The fiberglass was divided into the six groups depending on the treatment received: no pretreatment, heat treatment at 400 °C, 10% HCl etching for 1 h and 3 h, and heat treatment at 400 °C plus 10% HCl etching for 1 h and 3 h. For heat treatment, the fiberglass was placed in a muffle furnace and heated to 400 °C at a rate of 10 °C min−1, and incubated for 1 h at 400 °C. For acid treatment, the fiberglass was immersed in 10% HCl (mass fraction) for different time and then flushed with distilled water to remove chloride ions. For mixed treatment of heat and acid, after being acid treated, the fiberglass was heat treated followed by being dried. All pretreated fiberglass samples were silane-coated by immersing them in 2% KH570 for 1 h, and then dried at 100 °C for 1 h until silanization. They were labeled by Group a–f, orderly. The detailed reaction conditions and corresponding results are summarized in Table 1.| Group | Treatment condition of fiberglass | Breaking strength (N) |
|---|---|---|
| a | KH570 | 316.60 ± 5.92 |
| b | 400 °C + KH570 | 322.58 ± 9.87 |
| c | HCl for 1 h + KH570 | 297.32 ± 7.31 |
| d | HCl for 3 h + KH570 | 220.00 ± 8.49 |
| e | 400 °C + HCl for 1 h + KH570 | 293.50 ± 4.90 |
| f | 400 °C + HCl for 3 h + KH570 | 237.04 ± 7.56 |
Preparation of fiberglass-reinforced photocurable resin pile
Firstly, the resin matrix was prepared as following: the Bis-GMA, TEGDMA, DMAEMA, and CQ were weighed at a mass ratio of 78.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. These reagents were mixed, stirred at a constant temperature of 50 °C for 1 h, and stored away from light until use. Then, all pretreated fiberglass samples of Group a–f was immersed in the resin matrix and presoaked for 4 h. Furthermore, it was squeezed into a 25 mm × 2 mm × 2 mm post homemade abrasive tool. A dental curing light lamp (light intensity, 1250 mW cm−2) irradiated the surface of the abrasive tool for 60 s extremely. The productions were labeled by Group A–F, orderly. The detailed reaction conditions and corresponding results are summarized in Table 2.
0.5. These reagents were mixed, stirred at a constant temperature of 50 °C for 1 h, and stored away from light until use. Then, all pretreated fiberglass samples of Group a–f was immersed in the resin matrix and presoaked for 4 h. Furthermore, it was squeezed into a 25 mm × 2 mm × 2 mm post homemade abrasive tool. A dental curing light lamp (light intensity, 1250 mW cm−2) irradiated the surface of the abrasive tool for 60 s extremely. The productions were labeled by Group A–F, orderly. The detailed reaction conditions and corresponding results are summarized in Table 2.
| Group | Treatment condition of fiberglass | Flexural modulus | Flexural strength | Flexural load |
|---|---|---|---|---|
| A | KH-570 | 30.47 ± 1.47 | 631.77 ± 21.01 | 167.44 ± 6.74 |
| B | 400 °C + KH-570 | 37.70 ± 1.79 | 770.83 ± 16.98 | 196.53 ± 6.80 |
| C | HCl for 1 h + KH-570 | 34.77 ± 2.74 | 632.60 ± 28.17 | 171.53 ± 8.89 |
| D | HCl for 3 h + KH-570 | 34.16 ± 5.66 | 628.26 ± 19.64 | 167.55 ± 5.24 |
| E | 400 °C + HCl for 1 h + KH-570 | 35.47 ± 2.98 | 698.25 ± 25.35 | 180.34 ± 7.48 |
| F | 400 °C + HCl for 3 h + KH-570 | 32.89 ± 1.67 | 644.90 ± 24.34 | 166.04 ± 8.52 |
Characterizations
The morphology the samples were obtained using a scanning electron microscope (SEM, LEO1530VP, Carl Zeiss AG Germany; Hitachi, S-4800). The Fourier transformation infrared spectra (FTIR) were recorded on a Nicolet 6700 FT-IR spectrometer (USA). Thermogravimetric analyses (TGA) were conducted with a Perkin-Elmer thermoanalyzer instrument (USA), with 40 mL min−1 of nitrogen protection and heated at 10 °C min−1 until the temperature reached 600 °C.Measurement of fiberglass breaking strength
The fiberglass tensile strength was obtained at 23 °C and 60% humidity. Each of six treatment of Groups A–F (six groups, 10 per group) was stretched at 200 mm min−1 until the fiberglass fractured, and the breaking strength during being stretching and fracture were automatically collected by a computer. Statistical analysis and pairwise comparison were conducted through one-way analysis of variance (ANOVA), the SNK test and SPSS 16.0 software.Measurement of bending strength of FRP resin pile
The bending strength of FRP resin piles was measured by the three-point bending method. Specially, according to ISO 10477:92 standards,4 the two-point span was 20 mm, the load head diameter was 2 mm, and the loading speed was 1.0 mm min−1. The samples were continuously loaded until they fractured, and the loading records, flexural strength, and flexural modulus values were collected thereafter. Bending strength was calculated as 3FmaxL/2bh2, where Fmax is the maximum failure load, and L, b, and h are the span, width, and thickness of the pile, respectively. Flexural modulus (E) was calculated as SL3/4bh3, where L is the span, b is the specimen width, h is the sample thickness, and S is the slope. Statistical analysis and pairwise comparison were conducted using one-way ANOVA and the SNK test and SPSS 16.0.MTT colorimetric assay
Results
The structure and morphology of different surface treated fiberglass
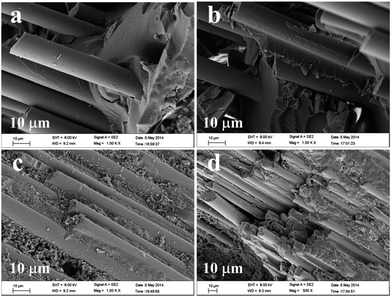
Breaking strength is the maximum force applied to the stretched fibers in the tensile and fracture process, which reflects the strength of the fiber. As shown in Table 1, the highest breaking strength is found in Group b compared to that of Group a, in which the fiberglass has been treated by heating to 400 °C. However, acid-treated fiber had a lower breaking strength and greater exposure to the acid resulted in further reduced fiber strength (Groups c and d), which suggests that the acid could destroy the fiber structure. Thus, as shown in Groups e and f, a fiberglass with an appropriate breaking strength could be adjusted by heat and acid treatment.The morphology of the obtained fiberglass with different surface treatments is characterized by scanning electron microscope (SEM) to evaluate the fiber structure. As shown in Fig. 1a–d of SEM images of Groups a, b, c and d, the fiberglass surface of Group d is the coarsest and that of Group b is the smoothest. Magnified SEM images of Groups a, b, c and d in figures further prove the above observation. As shown in Fig. 1e, part of fiberglass surface of Group a is marked by a layer with some bulges. However, there is rarely marker in the fiberglass surface of Group b (Fig. 1f). Furthermore, the fiberglass surface of Group c is filled with a marked layer with many bulges besides some nanoparticles (Fig. 1g). And these bulges are connected and thickened, which indicates the coarsest surface (Group d, Fig. 1h). The results show that heat treatment could remove the residual compound oxide on the fiber surface completely, whereas acid treatment could produce the surface damage of the material from acid etching.
 | ||
| Fig. 1 SEM images of Groups a (a), b (b), c (c) and d (d), and magnified SEM image of Groups a (e), b (f), c (g) and d (h). | ||
The thermal method was performed to quantify the absorbed organic materials. As shown in Fig. 2a, the weights loss is about 0.4% at 250–350 °C, which reveals that some organic materials absorb on the fiberglass surface. However, after being grafted by KH570, their weights loss happens at 350–550 °C and the weights loss is about 0.27%, 0.16%, 0.49% and 0.69% for Groups a, b, c and d, respectively (Fig. 2b–e). The results show that heat treatment goes against the absorption of KH570 due to the decrease of functional group, and acid treatment is helpful for the absorption of KH570 owe to the increased the number of hydroxyl ions. Therefore, in order to graft appropriate KH570 on fiberglass surface, the mixed treatment of heat and acid is necessary.
In order to evaluate the ability of KH570 grafting on fiberglass surface, FT-IR was used to estimate the functional group of fiberglass surface with surface treatments. As shown in Fig. 3, in the FT-IR spectra of Group a, the band at 3300 cm−1 of –OH stretching vibrations is strengthened through heat treatment, which reveals that heat treatment is helpful for the production of hydroxy groups. The hydroxy groups could react with KH570 to modified fiberglass surface. Meanwhile, in the FT-IR spectra of Groups c and e, the new bands at about 1640 and 1400 cm−1 appear after acid treatment or the mixed treatment of heat and acid. Generally, the bands at about 1600 and 1400 cm−1 are attributed to characteristic asymmetric and symmetric stretching vibrations of COO–, respectively. Therefore, the occurrence of the band at 1640 and 1400 cm−1 proves the production of carboxyl groups on the fiberglass surface, which is more capable to the KH570 grafting on fiberglass surface than that of hydroxy groups. Thus, acid treatment prefers to graft KH570 on fiberglass surface.
The effect of different fiberglass surface treatments on mechanical property of FRP resin pile
Fig. 4 and Table 2 list the main mechanical properties of FRP resin piles fabricated with different surface treatments. Group B was found to have the best mechanical properties, with a bending strength of 770.83 ± 6.98 MP. The results may be related to the removing surface wetting agent and increasing graft rate with KH570 through heat treatment, which results to strengthen the combination of the fiber–resin interface and improve its mechanical properties. However, the flexural modulus, flexural strength, and bending loads of Groups C, D, E, and F after acid treatment were reduced compared to the group that received heat treatment alone (Group B). Furthermore, acid exposure for a longer duration (3 h) resulted in a more obvious decline in these values; these results may be related to the poorer mechanical properties caused by the surface damage of the material from acid etching.The morphology of the samples after the three-point bending in the FRP resin piles was observed by SEM. Fig. 5 shows the mixed architecture with both resin and pulled out fibers of Groups A–D. When the fiberglass was not treated (Fig. 5a), in the FRP resin piles of Group A, there is few resin matrix attached to the resin matrix of the fiber surface and a gap between the resin matrix and the fiber could be found (labeled by arrow). However, the fiberglass was treated by heat or acid (Fig. 5b–d), clear resin matrix could adhere to the fiber surface of Groups B, C, and D. Specially, the fracture in Group D is neater than that in the other groups, and the fiber and resin show a more homogeneous appearance in the fractured region.
Cytotoxicity
The blank group was set as the negative control group, and the laboratory fabricated and RTD piles were chosen as the experimental group. Cytotoxicity was performed at days 1, 3, 5 and 7 of culture to evaluate the cytotoxicity. As shown in Fig. 6, the OD that dramatically increased with culture time, showing that the different treatments had no inhibitory effect on cell proliferation. Furthermore, the OD values between the groups did not significantly differ on any of the evaluated culture days (p > 0.05). | ||
| Fig. 6 Cytotoxicity assay after days 1, 3, 5, and 7. Data are represented as mean ± SD (n = 3). SD: standard deviation; OD: optical density. | ||
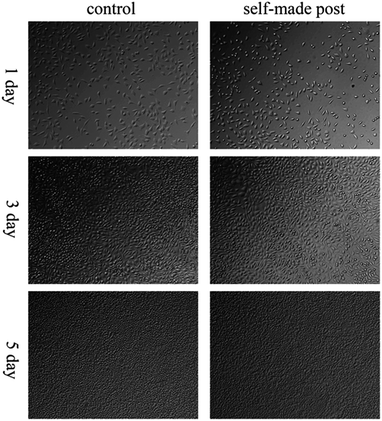
Fig. 7 shows the cell count of the control and laboratory-made pile groups. As shown in the figure, both counts showed a significant increase with incubation time, showing that the laboratory-made pile did not inhibit cell proliferation. Fig. 8 shows the cells on the laboratory-made pile group is good state and sprawl on the surface pile well no matter on the condition of bright and dark field. Furthermore, with the increasing of incubation time, more and more cells adhere to the pile, which implies the good biocompatibility of our laboratory-made pile. Fig. 9a shows the cell state on the laboratory-made pile group, which reveals the cells sprawl on the surface pile well. The enlarged SEM image (Fig. 9b) finds that the subtle cell parapodium stretches out freely. The result indicates that the laboratory-made high-strength glass fiber light-cured resin pile materials did not damage on cell morphology, growth, and proliferation, and has good cell compatibility.
Discussion
Many studies show that the mechanics performance of resin mainly depends on the properties of the fiber, the content of the resin matrix,18 and the interfacial bonding strength of the fiber and the resin.19 Of these, interface bonding strength is the most important.20 This experiment aimed to compare the effect of different surface treatments on the interface bonding ability and the properties of FRP resin piles. As fiber and resin are two different materials with different chemical properties, the fiber surface is often coated with a layer of organic wetting agent to keep clustery for weaving in the production process. The wetting agent reduces the interface bonding performance, thereby reducing the mechanical properties of the composite. Therefore, heat is used to remove the original compound from the surface of the fiber. Li et al.21 reported 450 °C to be the optimal temperature for heat treatment, and that the residual compound oxide on the fiber surface can be complete removed by heat treatment for 1 h. However, heat tends to damage and roughen the surface of the glass fiber, and the resultant surface cavities on the fiber surface cannot be completely filled by the resin because of its viscosity; therefore, a coupling agent is used for the anchor effect, i.e., to improve the interface properties of the composite materials. Therefore, this experiment adopted 400 °C + KH570 to process glass fiber, and as shown in Table 1, the mechanical properties of this group are obviously improved.Silane coupling agents (Y-R-Si-X3) are one of the most widely used substances for processing FRP resin. Here, R, Y, and X are alkylidene, organic functional groups that can react with resin and undergo hydrolysis, and the silanol generated through hydrolysis can be combined with inorganics.10,22,23 As KH570 has better double bonds and flexible long chains, which can significantly improve resin–fiber adhesion, it is widely used to modify the surface of the glass fiber and to prepare FRP resin piles. González-Benito et al. reported that etching heat-treated fiber with 10% HCl can increase the number of hydroxyl ions at the surface, improve surface energy to enhance the grafted rate of KH570, and increase the graft ratio with additional HCl treatment time.15 Therefore, the purpose of this experiment was to increase the fiber surface grafting ratio of KH570 using high temperature and acid treatment, thereby improving the surface energy and enhancing the fiber–resin interfacial binding force and improve the overall mechanical properties of the FRP resin piles. However, the bending strength of FRP resin fabricated using heat and acid treatment was lower than that fabricated using high temperature alone. Furthermore, increase in acid exposure obviously decreased the FRP resin piles strength. There were no statistically significant differences in the strength between the test and control groups, indicating that HCl treatment can increase the grafted rate of silane coupling agent but cannot significantly improve the mechanical properties of the FRP resin piles. Sever et al. found that the tensile strength, bending strength, interlaminar shear strength of FRP resin piles made of glass fiber and different concentration of HCl treatment were decreased compared to FRP resin piles that did not receive acid treatment.11 This is mainly due to the excess surface treatment, which damages the glass fiber and decreases the monofilament intensity, as evidenced by the fiber breaking strength observed in our study. Thus, although the acid treatment improves the fiber–resin interface binding ability, it does not compensate for the damage caused to the fiber, which is weakened and consequently results in reduced mechanical properties in the composite.
Similar studies have suggested that if the interface bonding is too weak, fibers will be pulled out from the matrix and they do not strengthen the interface. On the other hand, if the composite interface bonding is too strong, the composite materials will present a brittle fracture as the interface fails to relax the stress. The SEM results of our study revealed different fractures in all the groups. The control group showed an adhesive interface with fiber portions that pulled out from the matrix showing no obvious resin matrix adhesion, suggesting weak interface bonding strength and inability to effectively transfer load from the resin to the fiber, consequently having the lowest three-point bending strength among all the groups. In the acid-treated groups, the fiber and resin had both fractured. There was no interface debonding, and the cross-section was neat without obvious fiber pull-out and the specimen showed a brittle fracture, suggesting an excessively strong interface bond, which was higher than the strength of the composite materials, resulting in reduced bending strength. In the heat-treated groups, interface debonding and matrix fracture can both be seen along with adherence of the resin matrix on the fiber surface which was pulled out from the matrix, illustrating a moderate interface bond, as a result of which the bending strength is the highest.
The cytotoxicity test is a method used to evaluate the potential cytotoxicity of dental materials and medical devices or extracts, and cytotoxicity is one of the most important indices in biological evaluation.24–26 The MTT method is a colorimetric analysis method to rapidly evaluate cell proliferation and cytotoxicity, and it is the standard method to detect cytotoxicity. Meanwhile, it is also considered an important index to evaluate the toxicity of medical equipment. Here, L929 cells were chosen to complete the cytotoxicity test, which have strong proliferative ability and are sensitive to environmental factors.27 The results revealed a dramatic increase in the OD and cell count in the laboratory-made pile group over time; however, there was no significant difference in these parameters compared to the control group and RTD group, suggesting that the laboratory-made pile group did not have obvious cell toxicity. The possible reason for this finding is that the organic infiltration agent on the surface of self-made pile fibers were the most removed after high-temperature sintering, resulting in a considerably lower cytotoxicity. In general, the experimental results show that the self-made high-strength glass fiber light-cured resin matrix has good biocompatibility and meets the basic requirements for biological applications, which confirmed the applicability of the laboratory-made FRP resin piles.
Conclusion
High-strength fiberglass is subjected to different types of treatment: none, heat treatment at 400 °C, 10% HCl for 1 and 3 h, and heat plus 10% HCl etching for 1 and 3 h. Then, these groups are immersed in a laboratory-prepared photocurable resin substrate after KH570 treatment. We found that 400 °C and KH570 treatment yielded optimum stability and strength. High temperature aided in removing the sizing agents on the fiberglass surface, leading to more exposed groups and enhancing its combination with KH570 and mechanical properties. Furthermore, acid treatment reduced the performance of the FRP resin pile. MTT assay revealed no significant differences between our fabricated piles and the commercial piles. Moreover, FRP resin piles did not affect cell multiplication and the laboratory-made high-strength glass fiber light-cured resin piles have good cell compatibility.Acknowledgements
The work was supported by The Corpus of the National “863” Program (2012AA030309), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, 2014-37).Notes and references
- A. Lamichhane, C. Xu and F. Q. Zhang, J. Adv. Prosthodont., 2014, 6, 60 CrossRef PubMed.
- E. N. Brown, A. K. Davis, K. D. Jonnalagadda and N. R. Sottos, Compos. Sci. Technol., 2005, 65, 129 CrossRef CAS PubMed.
- K. Palanikumar, L. Karunamoorthy and R. Karthikeyan, Mater. Des., 2006, 27, 862 CrossRef CAS PubMed.
- C. Goracci and M. Ferrari, Aust. Dent. J., 2011, 56, 77 CrossRef PubMed.
- G. Bateman, D. N. J. Ricketts and W. P. Saunders, Br. Dent. J., 2003, 195, 43 CrossRef CAS PubMed.
- V. R. Novais, P. S. Quagliatto, A. D. Bona, L. Correr-Sobrinho and C. J. Soares, Indian J. Dent. Res., 2009, 20, 277 CrossRef PubMed.
- F. R. Tay and D. H. Pashley, Journal of Endodontics, 2007, 33, 391 CrossRef PubMed.
- B. Akkayan and T. Gulmez, J. Prosthet. Dent., 2002, 87, 431 CrossRef.
- S. O. Hedlund, N. G. Johansson and G. Sjogren, J. Oral Rehabil., 2003, 30, 1036 CrossRef.
- S. J. Park and J. S. Jin, J. Polym. Sci., Polym. Phys. Ed., 2003, 41, 55 CrossRef CAS PubMed.
- K. Sever, M. Sarikanat, Y. Seki, V. Cecen and I. H. Tavman, J. Mater. Sci., 2008, 43, 4666 CrossRef CAS.
- H. Z. Li, Y. X. Jia, S. F. Wan, Q. Xiang, C. C. Han, G. Mamtimin, Y. C. Han and L. J. An, Polym. Compos., 2008, 29, 964 CrossRef CAS PubMed.
- S. F. Luan, H. Z. Li, Y. X. Jia, L. J. An, Y. C. Han, Q. Xiang, J. Zhao, J. X. Li and C. C. Han, Polymer, 2006, 47, 6218 CrossRef CAS PubMed.
- L. Sun, Y. Jia, F. Ma, J. Zhao and C. C. Han, Macromol. Mater. Eng., 2008, 293, 194 CrossRef CAS PubMed.
- J. González-Benito, J. Baselga and A. J. Aznar, J. Mater. Process. Technol., 1999, 93, 129 CrossRef.
- R. L. Jones and D. Betz, J. Mater. Sci., 2004, 39, 5633 CrossRef CAS.
- V. Tomao, A. Siouffi and R. Denoyel, J. Chromatogr. A, 1998, 829, 367 CrossRef CAS.
- T. Williamson, APA engineered wood handbook, McGraw Hill Professional, 2002 Search PubMed.
- C. Hu, L. Q. Shao, L. L. Wang, S. Y. Zhou and J. Ai, Key Eng. Mater., 2012, 519, 269 CrossRef CAS.
- M. Chieruzzi, S. Pagano, M. Pennacchi, G. Lombardo, P. D'Errico and J. M. Kenny, J. Dent., 2012, 40, 968 CrossRef CAS PubMed.
- Z. F. Li and E. Ruckenstein, J. Colloid Interface Sci., 2002, 251, 343 CrossRef CAS PubMed.
- T. W. H. Wang, F. D. Blum and L. R. Dharani, J. Mater. Sci., 1999, 34, 4873 CrossRef CAS.
- V. Cech, N. Inagaki, J. Vanek, R. Prikryl, A. Grycova and J. Zemek, Thin Solid Films, 2006, 502, 181 CrossRef CAS PubMed.
- A. Wennberg, I. A. Mjor and A. Hensten-Pettersen, J. Biomed. Mater. Res., 1983, 17, 23 CrossRef CAS PubMed.
- C. T. Hanks, J. C. Wataha and Z. Sun, Dent. Mater., 1996, 12, 186 CrossRef CAS.
- J. C. Wataha, J. Prosthet. Dent., 2001, 86, 203 CrossRef CAS PubMed.
- J. S. Kwon, S. B. Lee, C. K. Kim and K. N. Kim, Acta Odontol. Scand., 2012, 70, 597 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra08964f |
| This journal is © The Royal Society of Chemistry 2015 |