Modulation of bovine serum albumin fibrillation by ester bonded and conventional gemini surfactants
Zahid Yaseen*a,
Sayeed Ur Rehmanb,
Mohammad Tabishb,
Aabid H. Shallaa and
Kabir-ud-Dinc
aDepartment of Chemistry, Islamic University of Science and Technology, Pulwama 192122, India. E-mail: zahid.yaseen@islamicuniversity.edu.in; zadn521@gmail.com; Tel: +91-9797264038
bDepartment of Biochemistry, Faculty of Life Sciences, Aligarh Muslim University, Aligarh-202002, India
cDepartment of Chemistry, Aligarh Muslim University, Aligarh-202002, India
First published on 29th June 2015
Abstract
Protein fibrillation has been associated with various neurological disorders. Knowledge about molecular mechanisms of protein aggregation modulators is potentially helpful for therapeutic purposes. In order to find out the key strategies for promotion and inhibition of protein fibrillation and to create better functional designs, we have studied the effect of gemini surfactants on the bovine serum albumin fibrillation. The ThT fluorescence emission spectrum indicates disintegration of BSA fibrils in presence of m-C2-m gemini surfactants (conventional). However, enhancement in BSA fibril formation takes place in presence of m-E2-m diester bonded gemini surfactants. Although, with increase in tail length of m-E2-m gemini surfactant, slight disintegration of fibrils also occurs. Circular dichroism data suggest decrease in the β-content of the BSA fibrils in presence of m-C2-m surfactant, while as increase in β-content of fibrils in presence of m-E2-m surfactant. FTIR, TEM, and confocal microscopic results also show that m-C2-m disintegrates and m-E2-m prompts the fibrillation. The electrostatic/hydrogen bonding/hydrophobic balance play important roles in determining the BSA fibrillation and disintegration. Micelles of m-C2-m surfactants disrupt the hydrogen bonding in between the β-strands, hence result in fragmented fibrils. Presence of diester group in m-E2-m surfactant forms hydrogen bonds in between the β-strands resulting in enhancement of fibril formation.
1 Introduction
Self aggregation of proteins and peptides leads to different morphological oligomers under various biophysical circumstances. This aggregation results in fibril formation.1–4 Protein self-assembly have been closely associated with various neurodegenerative diseases such as prion, Parkinson's, Huntington's and Alzheimer's.5,6 Appearance of amyloid β-peptide (Aβ) in the patients is feature of Alzheimer's disease. A strong correlation between the appearance of fibrillar plaques and disease progression has been shown, and also suggested that early intermediate aggregates are more toxic.7,8 Presently, no effectual treatment can avoid neurodegenerative diseases in humans, and existing drugs can only slightly assuage the symptoms.9Amyloid fibrils are thermodynamically stable,10 and hence the conversion of these fibrils into small fragments does not occur of its own. Therefore, in the recent past several approaches have been explored to break, destabilize and/or disrupt amyloid fibrils.11 Ultrasonification,12 high temperature (above 100 °C) and pH change result in fibril destruction.13,14 Electrostatic repulsive force needed to hold neighboring proto-fibrils together have been blocked by addition of strong ionic liquids, leading to destabilization of amyloid fibrils.15 Several studies have also been devoted to the effect of lipids on Aβ aggregation pathway,16,17 as in vivo lipids are known to be associated with amyloid fibrils.18,19 On the same lines, surfactants like SDS (sodium dodecylsulphate) and LiDS (lithium dodecylsulphate) have been chosen as model systems for studying amyloidogenic co-aggregate intermediate forms, as these surfactants favor β-rich structures.20 Ruhs et al.21 studied the interaction of sulphonic acid polyethylene glycol (surfactant) with amyloid fibrils. The synergistic interaction between the fibrils and polyethylene glycol chains reduces the entropy of amyloid fibril structure and converts it into amorphous globular ending structure. Sabate et al.22 have reported that alkylammonium surfactants below their cmc (critical micelle concentration) favor fibril formation and above the cmc can delay Aβ amyloid fibril formation, however, no idea about disintegration of fibrils. Han et al.9 proposes that gemini surfactant, C12C6C12Br2 – hexamethylene-1,6-bis(dodecyldimethylammonium bromide), has stronger interaction with amyloid fibrils based on the thermodynamical profile. Cao et al.23 studied the effect of surfactant concentration on the dynamics of Aβ fibrillogenesis. All these studies suggest that surfactants represent one of the important categories of molecules which modulate the amyloid fibrils. In spite of various studies, much remain to be elucidated regarding the structure of surfactant molecules, especially of gemini surfactants, on the inhibition and formation of amyloid fibrils.
Gemini surfactants possess two hydrophilic head groups covalently connected by a spacer. They have lower cmc and stronger ability to self-aggregate as compared to conventional surfactants. The motivations in picking the cationic gemini surfactants for modulating protein aggregation are (a) their structural analogy to complex cationic lipids, hence, represent the molecular aspect of membranes in amyloid formation24 (b) they can serve as drug delivery carriers.25 Recently, our group have revealed the gene transfection efficiency of biocompatible diester bonded gemini surfactants.26 These surfactants also show strong ability in stabilizing the aggregated form of bile salts and organic hydrotopes.27,28 In the present study, we have chosen two sets of gemini surfactants (a) m-E2-m (E2 represents diester spacer) and (b) m-C2-m (C2 represents conventional spacer with two methylene groups), where m = 12, 14 and 16, depicts the number of carbon atoms in surfactant tail. Observably, the chosen surfactants have different structure as shown in Scheme 1. The main objective is to reveal the mode of binding of these surfactants with BSA fibrils. Secondly, the effects of alkyl tail length and the nature of spacer in surfactants on the BSA fibril modulation are seen. To get a deeper perceptive of the interaction, a series of techniques have been used. These studies shed light on the hydrophilic/hydrophobic effects which play important role in bringing about reflective perturbations in secondary structure of BSA fibrils. Also, it is quite interesting to analyze the effect of diester groups in the spacer of m-E2-m gemini surfactants on the BSA fibrils.
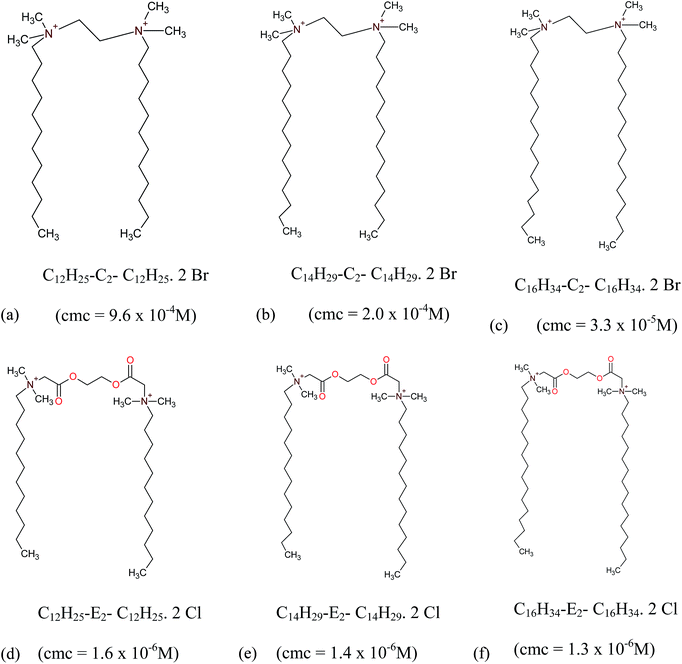 | ||
| Scheme 1 Structure of surfactants (a–c) m-C2-m (d–f) m-E2-m.28–31 | ||
2 Experimental section
2.1 Materials
Bovine Serum Albumin (BSA) and thioflavin T (ThT) were purchased from Sigma Chemical Co. (St. Louis, USA) and used as received.2.2 Measurements
3 Results
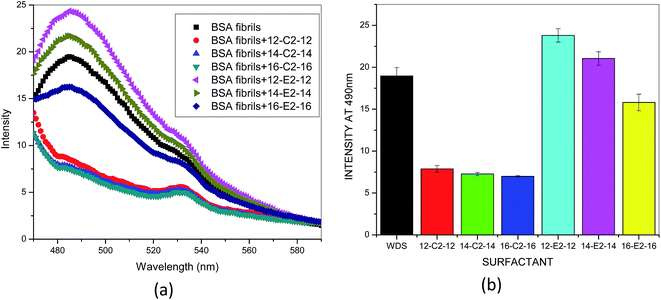
ThT has been widely used to study fibril formation in a number of proteins aggregates including α-synuclein, Aβ amyloids, lysozyme, and β2m. ThT molecules bind to the “channels” formed in β-sheets due to the interactions between the backbone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from one strand and N–H from the other strand that form the fibrils.43 The extent of ThT binding to amyloid samples depends on the accessibility of these binding grooves. Once β sheet starts forming, ThT binding is initiated, and it increases in proportion to the content of β-sheet in the system. The aggregation of Aβ fibrils in the absence of surfactant shows sigmoid ThT fluorescence trace, Fig. 1a. Gemini surfactants m-C2-m show significant decrease in the ThT fluorescence intensity. The order of decrease in the intensity of ThT is: 12-C2-12 < 14-C2-14 < 16-C2-16, although the difference in lowering of fluorescence intensity between these gemini surfactants is very less (Fig. 1b). The reduction observed in the ThT intensity implies that there is a decrease in fibrillar species present in solution. Within this set of surfactants, 16-C2-16 represents the best one in disintegration of fibrillar species. However, the gemini surfactants m-E2-m show interesting results, in which 12-E2-12 and 14-E2-14 increase the binding grooves for ThT binding on amyloid fibrils, which result in increase in the ThT fluorescence intensity as compared to that of fibrils without surfactants (Fig. 1b). Furthermore, with increase in the hydrophobic tail length of m-E2-m surfactants, as in case of 16-E2-16, there is 11% reduction in the ThT fluorescence intensity with respect to the fibrils without surfactant.
O from one strand and N–H from the other strand that form the fibrils.43 The extent of ThT binding to amyloid samples depends on the accessibility of these binding grooves. Once β sheet starts forming, ThT binding is initiated, and it increases in proportion to the content of β-sheet in the system. The aggregation of Aβ fibrils in the absence of surfactant shows sigmoid ThT fluorescence trace, Fig. 1a. Gemini surfactants m-C2-m show significant decrease in the ThT fluorescence intensity. The order of decrease in the intensity of ThT is: 12-C2-12 < 14-C2-14 < 16-C2-16, although the difference in lowering of fluorescence intensity between these gemini surfactants is very less (Fig. 1b). The reduction observed in the ThT intensity implies that there is a decrease in fibrillar species present in solution. Within this set of surfactants, 16-C2-16 represents the best one in disintegration of fibrillar species. However, the gemini surfactants m-E2-m show interesting results, in which 12-E2-12 and 14-E2-14 increase the binding grooves for ThT binding on amyloid fibrils, which result in increase in the ThT fluorescence intensity as compared to that of fibrils without surfactants (Fig. 1b). Furthermore, with increase in the hydrophobic tail length of m-E2-m surfactants, as in case of 16-E2-16, there is 11% reduction in the ThT fluorescence intensity with respect to the fibrils without surfactant.
We have also applied circular dichroism spectroscopy for exploring the secondary structural changes of BSA fibrils in presence of surfactants. Proteins dominant with α-content and α + β content proteins exhibit two negative peaks, one at 208 nm and other at 222 nm, while β-content proteins show a single negative peak between 215 nm and 222 nm. From Fig. 2, it is quite evident that BSA fibrils and BSA fibrils in presence of m-E2-m surfactants show only a single negative broad peak in between 212 nm to 220 nm, indicating the dominance of β-sheets. However, in presence of m-C2-m, the BSA fibrils show two negative peaks (208 nm and 223 nm), confirming the presence of α + β content. Table 1 summarizes the results of secondary structural changes in BSA fibrils in presence of different surfactants. The CD data depicts that BSA fibrils are rich in β-sheets. In case of BSA fibrils in presence of m-C2-m surfactants, there is decrease in the β-sheet and increase in α-helix. In presence of m-E2-m surfactants, there is increase in the β-sheet content of the BSA fibrils. These results suggest the disintegration of BSA fibrils in presence of m-C2-m surfactants while as m-E2-m assists fibril formation, which are in total agreement with fluorescence results.
| System | α-Helix (%) | β-Sheet (%) | Random (%) |
|---|---|---|---|
| BSA fibril | 04.33 | 59.15 | 20.22 |
| BSA fibril + 12-C2-12 | 22.11 | 09.33 | 18.11 |
| BSA fibril + 14-C2-14 | 23.09 | 06.55 | 12.13 |
| BSA fibril + 16-C2-16 | 27.33 | 06.50 | 12.14 |
| BSA fibril + 12-E2-12 | 05.11 | 61.55 | 18.22 |
| BSA fibril + 14-E2-14 | 05.14 | 60.33 | 19.11 |
| BSA fibril + 16-E2-16 | 14.12 | 56.44 | 12.22 |
FT-IR spectroscopy has been extensively used to analyze amyloid formation,44 as it enables secondary structural fluctuations from the initial monomer to the amyloid formation. A prominent peak at approximately 1654 cm−1 is characteristic feature of albumin proteins. This band can be attributed to the amide I modes of these predominantly α-helical proteins.45 Fig. 3 shows FTIR spectra of BSA fibrils and BSA fibril–surfactant complexes. BSA fibrils and complexes of BSA fibrils + m-E2-m surfactants does not show band in vicinity of 1650 cm−1. However, there is appearance of band near 1650 cm−1 in BSA fibrils + m-C2-m surfactant systems. These results are in agreement with CD results, which clearly indicate that there is increase in α-helical content in presence of m-C2-m surfactants.
 | ||
| Fig. 3 FT-IR spectrum of BSA fibrils incubated without (a) and with 16-E2-16 (b), 14-E2-14 (c), 12-E2-12 (d), 16-C2-16 (e), 14-C2-14 (f), and 12-C2-12 (g). | ||
Transmission electron microscopy (TEM) was used to verify the effect of the surfactants on the fibrillar morphology, during the formation and disintegration process. The BSA fibrils and fibrils treated with surfactant were subjected to negative TEM analysis. Fig. 4 represents the morphology of BSA fibrils. The length of the fibrils was measured and found that more than 65% of populations have an average length of 1000 ± 350 nm with average diameter of 22 ± 4 nm. BSA fibrils treated with m-C2-m type of surfactants show disappearance of the fibrils. More specifically, in 14-C2-14 and 16-C2-16 there is almost complete absence of fibrils, however, the fibrillar density is less in case of 12-C2-12 (Fig. 4b). Various research groups have used negative-stain TEM to estimate the length or width of the Aβ fibrils, which was found to be in quite good agreement with cryo-EM technique.46–48 Considering that the average length and width of 12-C2-12 treated BSA fibril resulted in shorter but similar thickness as that of surfactant free BSA fibrils, indicates these are fragmented type of fibrils. All the surfactants from m-E2-m category show the presence of fibrils when treated with BSA fibrillar solution. 12-E2-12 treated BSA fibrillar solution shows dense fibrillar formation as represented in Fig. 4c. With increase in the tail length, the density of fibrils decreases as in case of 14-E2-14 and 16-E2-16 (Fig. 4d and e). The TEM results are well supported by the confocal microscopy images. The presence of BSA fibrils and its morphology in absence and presence of gemini surfactants was also studied via confocal microscopy. A green fluorescence was observed when the ThT-stained fibrils were excited at 450 nm using an appropriate filter. CM images initially show ribbon-like fibrils in case of BSA, whereas in presence of m-C2-m show almost complete disappearance of fibrils (Fig. 5). The BSA fibrils treated with 12-E2-12 surfactant show enhancement in the fibrillar density, and elongated fibrils from morphological point of view. In case of 14-E2-14 surfactant, the fibrillar density decreases and is scattered ribbon-like in comparison to that of 12-E2-12, although more than that of BSA fibrils without surfactants. In 16-E2-16 treated BSA fibrils, the density of fibrils is less. These results are in total agreement with that of ThT fluorescence and CD.
 | ||
| Fig. 4 TEM images of BSA fibrils incubated without (a) and with 12-C2-12 (b), 12-E2-12 (c), 14-E2-14 (d), 16-E2-16 (e). | ||
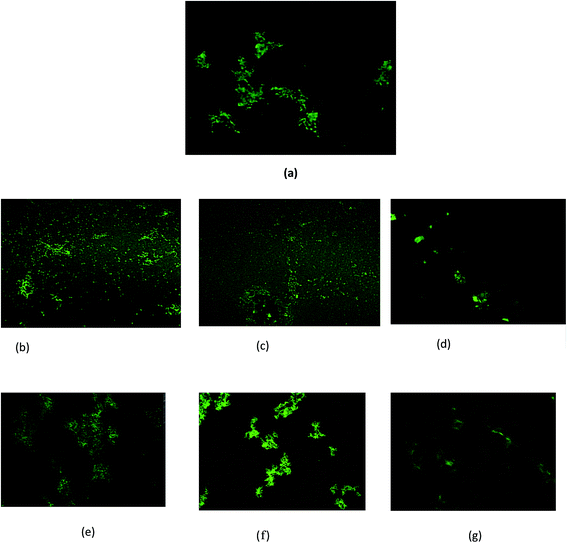 | ||
| Fig. 5 Confocal microscopic images of BSA fibrils incubated without (a) and with 12-C2-12 (b), 14-C2-14 (c), 16-C2-16 (d), 12-E2-12 (e), 14-E2-14 (f), and 16-E2-16 (g). Scale bar of 500 nm. | ||
4 Discussions
Herein, we demonstrate that m-C2-m gemini surfactants inhibit the BSA fibril formation. Among m-C2-m category, 16-C2-16 represents the most effective in disintegrating the fibril formation. In each case the concentration of surfactant is above than that of cmc, and exist as prolate ellipsoidal in the chosen concentration range.49,50 From TEM analysis it is evident that the BSA fibrils in presence of 12-C2-12 surfactants get shorter in length, hence results in fragmented β-fibrils. Also CD results suggest that there is decrease in β-sheet content in presence of m-C2-m surfactants. In order to explain this phenomenon, we have to focus on the mechanism of fibril formation by increasing temperature treatment. One of the major theory on protein fibrillation states that the protein only partially unfolds51,52 and then forms inter-aligned β-sheets between the partially unfolded patches of protein.53,54 This conformational fluctuation initiates the protein fibril formation. Another theory states that protein fibrillation at high temperature involves not only denaturation but also hydrolysis of the protein into peptide fragments, which then assemble into the fibrils.55–57 The secondary structure of the α-helix is formed by the hydrogen bond between the NH and CO groups of the same strand whereas the β-strands form hydrogen bonds with other β-strands. These β-strands stabilize each other through intermolecular hydrogen bonding. At pH 7.4, BSA fibrils carry net negative charge; hence the micelles of dicationic gemini surfactants m-C2-m get adsorbed on the fibrils via electrostatic interaction. The positively charged head groups of the gemini surfactants intercalate in between the NH terminus and CO terminus of the other strand, hence inhibits their involvement in hydrogen bonding. Thus, m-C2-m micelles pull BSA fibril sheets apart, results in breaking long, thick fibrils into short, fragmented pieces. These results also support that fibrillation of protein on heat treatment occurs via hydrolysis of the protein into peptide fragments, which then aggregate into fibril formation. With increase in the tail length of gemini surfactants their self-aggregation ability increases which, inturn, increases their ability to interact with the hydrophobic tails of the peptide molecules. These findings reveal that both electrostatic as well as hydrophobic forces are a prerequisite for disaggregation of fibril formation but electrostatic interaction plays a primary role. BSA fibrils in presence of m-E2-m surfactants show interesting results. Gemini surfactants 12-E2-12 and 14-E2-14 tend to prompt the BSA fibril formation, 16-E2-16 results in slight disintegration of fibrils. The diester dicationic gemini surfactants (m-E2-m) micelles bind on the BSA fibrils due to electrostatic interactions. However, the two CO groups in the spacer part of gemini surfactant get involved in the hydrogen bonding between the β-strands, hence holding them intact. Therefore, m-E2-m surfactants aids in the BSA fibril formation. With increasing hydrophobicity (by increase in the surfactant tail length, as in case of 16-E2-16), the fibrils get slightly destabilized. The basis for the slight disintegration of fibrillar assembly by 16-E2-16 compared to the others in the m-E2-m series could be ascribed to two factors – lower cmc value30 which is due to its longer hydrophobic tail and larger ellipsoidal dimensions. A comparison of the ellipsoidal dimensions of 16-E2-16 (semi-major axis a = 50.5 Å, semi-minor axis, b = c = 18.2 Å) micelles compared to 12-E2-12 micelles (a = 32.6 Å, b = c = 13.4 Å)49 indicates that the long hydrocarbon chain would allow better spatial interaction with the hydrophobic portion of the BSA fibrils thus making it more effective in causing a breakdown of the fibrillar assembly of BSA. Therefore, in m-E2-m gemini surfactants, hydrogen bonding plays a crucial role in prompting the fibril formation, although this efficacy gets faded with increase in hydrophobicity (Scheme 2).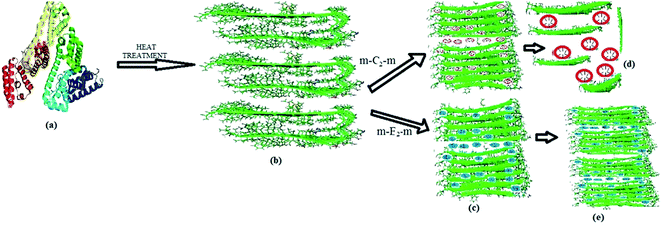 | ||
| Scheme 2 Mechanism of the gemini surfactants disintegration/formation of the BSA fibril. (a) BSA protein (PDB: 1E7I) (b) BSA fibrils (c) binding of the gemini surfactants onto the fibril surface. (d) Breaking down of a long fibril into fragmented fibrils. (e) Prompt the BSA fibril formation by m-E2-m surfactants. | ||
5 Conclusion
We have investigated the effect of gemini surfactants m-C2-m and m-E2-m on the BSA fibrils by using a broad range of biophysical techniques. All the results suggest that m-C2-m gemini surfactants destabilize the BSA fibrils, and m-E2-m surfactants prompt the fibrillation. Micelles of dicationic m-C2-m gemini surfactants get adsorbed on BSA fibrils via electrostatic and hydrophobic interactions. These micelles inhibit the hydrogen bonding in between β-strands, hence disintegrate them into small fragments. However, the presence of diester group in the spacer of gemini m-E2-m involves in hydrogen bonding between the β-strands and keeps them intact, hence aids in fibril formation. Increase in tail length, however, as in 16-E2-16, also tries to destabilize the β sheet framework.Acknowledgements
KUD is thankful to University Grants Commission (UGC) (New Delhi, India) for the BSR FACULTY FELLOWSHIP award. ZY and AHS are thankful to UGC for Research grant MRP-MAJOR-CHEM-2013-2226. We also thank the Advanced Instrumentation Research Facility, Jawaharlal Nehru University (New Delhi, India) for performing CD and confocal experiments, and Aligarh Muslim University for TEM facility.References
- C. M. Dobson, The structural basis of protein folding and its links with human disease, Philos. Trans. R. Soc. London, Ser. B, 2001, 356, 133–145 CrossRef CAS PubMed.
- C. M. Dobson, Protein misfolding, evolution and disease, Trends Biochem. Sci., 1999, 24, 329–332 CrossRef CAS.
- E. Gazit, The ‘correctly-folded’ state of proteins: Is it a metastable state?, Angew. Chem., Int. Ed., 2002, 41, 257–259 CrossRef CAS.
- N. K. Pandey, S. Ghosh and S. Dasgupta, Fibrillation in Human serum albumin is enhanced in the presence of copper(II), J. Phys. Chem. B, 2010, 114, 10228–10233 CrossRef CAS PubMed.
- F. Chiti and C. M. Dobson, Protein misfolding, functional amyloid, and human disease, Annu. Rev. Biochem., 2006, 75, 333–366 CrossRef CAS PubMed.
- J. Hardy and D. J. Selkoe, The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics, Science, 2002, 297, 353–356 CrossRef CAS PubMed.
- C. Haass and D. J. Selkoe, Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide, Nat. Rev. Mol. Cell Biol., 2007, 8, 101–112 CrossRef CAS PubMed.
- I. Benilova, E. Karran and B. De Strooper, The toxic Aβ oligomerand Alzheimer's disease: an emperor in need of clothes, Nat. Neurosci., 2012, 15, 349–357 CrossRef CAS PubMed.
- Y. Han, C. He, M. Cao, X. Huang, Y. Wang and Z. Li, Facile disassembly of amyloid fibrils using gemini surfactant micelles, Langmuir, 2010, 26, 1583–1587 CrossRef CAS PubMed.
- T. R. Jahn and S. E. Radford, The Yin and Yang of Protein Folding, FEBS J., 2005, 272, 5962–5970 CrossRef CAS PubMed.
- O. G. Jones and R. Mezzenga, Inhibiting, Promoting, and Preserving Stability of Functional Protein Fibrils, Soft Matter, 2012, 8, 876–895 RSC.
- E. Chatani, Y.-H. Lee, H. Yagi, Y. Yoshimura, H. Naiki and Y. Goto, Ultrasonication-Dependent Production and Breakdown Lead to Minimum-Sized Amyloid Fibrils, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 11119–11124 CrossRef CAS PubMed.
- A. Arora, C. Ha and C. B. Park, Insulin Amyloid Fibrillation at above 100 °C: New Insights into Protein Folding under Extreme Temperatures, Protein Sci., 2004, 13, 29–2436 Search PubMed.
- J. Dubois, A. A. Ismail, S. L. Chan and Z. A. Khan, Fourier Transform Infrared Spectroscopic Investigation of Temperature- and Pressure-Induced Disaggregation of Amyloid A, Scand. J. Immunol., 1999, 49, 376–380 CrossRef CAS.
- J. Adamcik and R. Mezzenga, Adjustable Twisting Periodic Pitch of Amyloid Fibrils, Soft Matter, 2011, 7, 5437–5443 RSC.
- I. C. Martins, I. Kuperstein, H. Wilkinson, E. Maes, M. Vanbrabant, W. Jonckheere, P. Van Gelder, D. Hartmann, R. D'Hooge, B. De Strooper, J. Schymkowitz and F. Rousseau, Lipids revert inert Aβ amyloid fibrils to neurotoxic protofibrils that affect learning in mice, EMBO J., 2008, 27, 224–233 CrossRef CAS PubMed.
- F. M. LaFerla, K. N. Green and S. Oddo, Intracellular amyloid-β in Alzheimer's disease, Nat. Rev. Neurosci., 2007, 8, 499–509 CrossRef CAS PubMed.
- A. Kuzyk, M. Kastyak, V. Agrawal, M. Gallant, G. Sivakumar, M. Rak, M. R. Del Bigio, D. Westaway, R. Julian and K. M. Gough, Association among amyloid plaque, lipid, and creatine in hippocampus of TgCRND8 mouse model for Alzheimer disease, J. Biol. Chem., 2010, 285, 31202–31207 CrossRef CAS PubMed.
- C. R. Liao, M. Rak, J. Lund, M. Unger, E. Platt, B. C. Albensi, C. J. Hirschmugl and K. M. Gough, Synchrotron FTIR reveals lipid around and within amyloid plaques in transgenic mice and Alzheimer's disease brain, Analyst, 2013, 138, 3991–3997 RSC.
- A. Abelein, J. D. Kaspersen, S. B. Nielsen, G. V. Jensen, G. Christiansen, J. S. Pedersen, J. Danielsson, D. E. Otzen and A. Gräslund, Formation of dynamic soluble surfactant-induced amyloid β peptide aggregation intermediates, J. Biol. Chem., 2013, 228, 23518–23528 CrossRef PubMed.
- P. A. Ruhs, J. Adamcik, S. Bolisetty, A. Sanchez-Ferrer and R. Mezzenga, A Supramolecular Bottle-Brush Approach to Disassemble Amyloid Fibrils, Soft Matter, 2011, 7, 3571–3579 RSC.
- R. Sabate and J. Estelrich, Stimulatory and inhibitory effects of alkyl bromide surfactants on β-amyloid fibrillogenesis, Langmuir, 2005, 21, 6944–6949 CrossRef CAS PubMed.
- M. Cao, Y. Han, J. Wang and Y. Wang, Modulation of fibrillogenesis of amyloid β(1–40) peptide with cationic gemini surfactant, J. Phys. Chem. B, 2007, 111, 13436–13443 CrossRef CAS PubMed.
- G. D. Henry and B. D. Sykes, Methods to study membrane protein structure in solution, Methods Enzymol., 1994, 239, 515–535 CAS.
- A. J. Kirby, P. Camilleri, J. B. F. N. Engberts, M. C. Feiters, R. J. M. Nolte, O. Soderman, M. Bergsma, P. C. Bell, M. L. Fielden, C. L. Garcıa Rodrıguez, P. Guedat, A. Kremer, C. McGregor, C. Perrin, G. Ronsin and M. C. P. van Eijk, Angew. Chem., Int. Ed., 2003, 42, 1448–1457 CrossRef CAS PubMed.
- Z. Yaseen, S. Rehman, M. Tabish and Kabir-ud-Din, Interaction between DNA and Cationic Diester Bonded Gemini Surfactants, J. Mol. Liq., 2014, 197, 322–327 CrossRef CAS PubMed.
- M. Akram, I. A. Bhat, Z. Yaseen and Kabir-ud-Din, Physicochemical investigation of novel biodegradable dicationic ester bonded m-E2-m gemini surfactants with bile salts: Insights from surface tension, dynamic light scattering and fluorescence, Colloids Surf., A, 2014, 444, 209–216 CrossRef CAS PubMed.
- M. Akram, I. A. Bhat and Kabir-ud-Din, Self-Aggregation of surfactant ethane-1,2-diyl bis(N,N-dimethyl-N-hexadecylammoniumacetoxy) dichloride: tensiometric, microscopic, and spectroscopic studies, J. Phys. Chem. B, 2015, 119, 3499–3509 CrossRef CAS PubMed.
- N. Fatma, W. H. Ansari, M. Panda and Kabir-ud-Din, Mixed micellization behavior of gemini (cationic ester-bonded) surfactants with conventional (cationic, anionic and nonionic) surfactants in aqueous medium, Z. Phys. Chem., 2012, 227, 133–149 Search PubMed.
- W. H. Ansari, N. Fatma, M. Panda and Kabir-ud-Din, Solubilization of polycyclic aromatic hydrocarbons by novel biodegradable cationic gemini surfactant ethane-1,2-diyl bis(N,N-dimethyl-N-hexadecyl-ammoniumacetoxy) dichloride and its binary mixtures with conventional surfactants, Soft Matter, 2013, 9, 1478–1487 RSC.
- Kabir-ud-Din, Z. Yaseen, V. K. Aswal and A. A. Dar, Rheological response and small-angle neutron-scattering study of diester bonded cationic biodegradable gemini surfactants in presence of different additives, Colloid Polym. Sci., 2014, 292, 3113–3125 CAS.
- N. Azum, A. Z. Naqvi, M. Akram and Kabir-ud-Din, Mixing behavior of conventional and cationic gemini surfactants, J. Dispersion Sci. Technol., 2008, 29, 711–717 CrossRef CAS PubMed.
- Gemini surfactants: synthesis, interfacial and solution-phase behavior, and applications, ed. R. Zana and J. Xia, Surf. Sci. Series, Marcel Dekker, New York, USA, 2003, vol. 713 Search PubMed.
- N. Gull, P. Sen, R. H. Khan and Kabir-ud-Din, Interaction of bovine (BSA), rabbit (RSA) and porcine (SPSA) serum albumins with cationic single chain/gemini surfactants. A comparative study, Langmuir, 2009, 25, 11686–11691 CrossRef CAS PubMed.
- B. Farruggia, F. Rodriguez, R. Rigatuso, G. Fidelio and G. Pico, The participation of human serum albumin domains in chemical and thermal unfolding, J. Protein Chem., 2001, 20, 81–89 CrossRef CAS.
- K. Flora, J. D. Brennan, G. A. Baker, M. A. Doody and F. V. Bright, Biophys. J., 1998, 75, 1084–1096 CrossRef CAS.
- R. Khurana, C. Coleman, C. Ionescu-Zanetti, A. K. Carter Sue and V. K. Grover, Mechanism of thioflavin T binding to amyloid fibrils, J. Struct. Biol., 2005, 151, 229–238 CrossRef CAS PubMed.
- L. Whitmore and B. A. Wallace, Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases, Biopolymers, 2008, 89(5), 392–400 CrossRef CAS PubMed.
- S. W. Provencher and J. Gloeckner, Estimation of globular protein secondary structure from circular dichroism, Biochemistry, 1981, 20(1), 33–37 CrossRef CAS.
- I. Vanstokkum, Estimation of protein secondary structure and error analysis from circular dichroism spectra, Anal. Biochem., 1990, 191(1), 110–118 CrossRef CAS.
- N. Sreerama and R. W. Woody, Estimation of protein secondary structure from circular dichroism spectra: comparison of contin, selcon, and cdsstr methods with an expanded reference set, Anal. Biochem., 2000, 287(2), 252–260 CrossRef CAS PubMed.
- N. Sreerama, S. Y. Venyaminov and R. W. Woody, Estimation of protein secondary structure from circular dichroism spectra: inclusion of denatured proteins with native proteins in the analysis, Anal. Biochem., 2000, 287(2), 243–251 CrossRef CAS PubMed.
- H. LeVine III, Thioflavin T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution, Protein Sci., 1993, 2, 404–410 CrossRef PubMed.
- I. D. L. Arada, N. Andraka, M. G. Pacios and J. L. R. Arrondo, A conventional and 2DCOS infrared approach to the kinetics of protein misfolding, Curr. Protein Pept. Sci., 2011, 12, 181–187 CrossRef.
- J. L. Arrondo, A. Muga, J. Castresana and F. M. GoñI, Quantitative studies of the structure of proteins in solution by Fourier transform infrared spectroscopy, Prog. Biophys. Mol. Biol., 1993, 59, 23–56 CrossRef CAS.
- L. A. Woods, G. W. Platt, A. L. Hellewell, E. W. Hewitt, S. W. Homans, E. Ashcroft and S. E. Radford, Ligand binding to distinct states diverts aggregation of an amyloid-forming protein, Nat. Chem. Biol., 2011, 7(10), 730–739 CrossRef CAS PubMed.
- M. Ahmed, J. Davis, D. Aucoin, T. Sato, S. Ahuja, S. Aimoto, J. I. Elliott, W. E. Van Nostrand and S. O. Smith, Structural conversion of neurotoxic amyloid-β(1–42) oligomers to fibrils, Nat. Struct. Mol. Biol., 2010, 17(5), 561–567 CAS.
- S. Chimon, M. A. Shaibat, C. R. Jones, D. C. Calero, B. Aizezi and Y. Ishii, Evidence of fibril-like β-sheet structures in a neurotoxic amyloid intermediate of alzheimer's β-amyloid, Nat. Struct. Mol. Biol., 2007, 14(12), 1157–1164 CAS.
- Kabir-ud-Din, Z. Yaseen, V. Kumar Aswal and A. A. Dar, Rheological response and small-angle neutron-scattering study of diester-bonded cationic biodegradable gemini surfactants in presence of different additives, Colloid Polym. Sci., 2014, 292(12), 3113–3125 CAS.
- S. De, V. K. Aswal, P. S. Goyal and S. Bhattacharya, Role of spacer chain length in dimeric micellar organization. small angle neutron scattering and fluorescence studies, J. Phys. Chem. B, 1996, 100(28), 11664–11671 CrossRef CAS.
- F. Chiti and C. M. Dobson, Amyloid formation by globular proteins under native conditions, Nat. Chem. Biol., 2009, 5, 15–22 CrossRef CAS PubMed.
- V. N. Uversky and A. L. Fink, Conformational constraints for amyloid fibrillation: the importance of being unfolded, Biochim. Biophys. Acta, 2004, 1698, 131–153 CrossRef CAS PubMed.
- C. E. MacPhee and C. M. Dobson, Chemical dissection and reassembly of amyloid fibrils formed by a peptide fragment of transthyretin, J. Mol. Biol., 2000, 297, 1203–1215 CrossRef CAS PubMed.
- F. Chiti, P. Webster, N. Taddei, A. Clark, M. Stefani, G. Ramponi and C. M. Dobson, Designing conditions for in vitro formation of amyloid protofilaments and fibrils, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 3590–3594 CrossRef CAS.
- S. Jordens, J. Adamcik, I. Amar-Yuli and R. Mezzenga, Disassembly and reassembly of amyloid fibrils in water–ethanol mixtures, Biomacromolecules, 2011, 12, 187–193 CrossRef CAS PubMed.
- C. Lara, J. Adamcik, S. Jordens and R. Mezzenga, General self-assembly mechanism converting hydrolyzed globular proteins into giant multistranded amyloid ribbons, Biomacromolecules, 2011, 77, 167–180 Search PubMed.
- C. Akkermans, P. Venema, A. J. van der Goot, H. Gruppen, E. J. Bakx, R. M. Boom and E. van der Linden, Peptides are building blocks of heat-induced fibrillar protein aggregates of β-lactoglobulin formed at pH 2, Biomacromolecules, 2008, 9, 1474–1479 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |