Effect of heat treatment on the lubricating properties of lithium lubricating grease
Pan Jiabao,
Cheng Yanhai* and
Yang Jinyong
School of Mechanical and Electrical Engineering, China University of Mining and Technology, Xuzhou, 221116, China. E-mail: chyh1007@cumt.edu.cn; Fax: +86-516-83590708; Tel: +86-150-05208612
First published on 17th June 2015
Abstract
Owing to the potential oxidation and thermal degradation of lithium lubricating grease when the grease is heated to improve its fluidity in the pumping process, fresh NLGI 3 lithium lubricating grease samples were heated to obtain experimental samples to simulate the static thermal degradation by using a drying oven. The microstructure and infrared spectra were studied by using field emission scanning electron microscopy (FESEM) and Fourier transform infrared spectroscopy (FTIR) techniques respectively. The rheological properties were estimated by a rotational rheometer and the tribological properties were evaluated on a four-ball testing machine. Finally, the action mechanisms of the effect of heat treatment on the tribological properties of lithium lubricating grease are discussed. The experimental results showed that the lithium lubricating grease samples exhibited better antifriction and antiwear properties after heat treatment at 120 °C, which is not obvious during 0–4 h, while the coefficient friction and extreme pressure progressively decreased during 4–24 h. Meanwhile, oxidative and thermal degradation do not proceed after heat treatment for 0–24 h according to the FESEM and FTIR results. Finally, it was confirmed that the variation of physical entanglement, which was caused by a short heat treatment, would vary the tribological properties of the lithium lubricating grease.
1. Introduction
Lubricating grease, a semi-solid colloidal dispersion system, consists of a thickener and base oil, and displays high-viscosity and weak fluidity properties.1,2 Therefore, a high pipe resistance would be shown when the grease is delivered by the centralized lubrication system, which is a disadvantage for the centralized lubrication system application. At present, only parts of lubricating greases, which have better fluidity, are delivered by centralized lubrication systems, such as NLGI 1, 0, 00 lubricating greases. However, NLGI 2 and NLGI 3 lubricating greases, which are widely used in industrial applications, are difficult to deliver by a centralized lubrication system due to their weak fluidity at room temperature. Li et al.3 detected that the grease displays weak fluidity in the experimental channel and a certain distance is required to let the flow develop fully. Delgado et al.4 found that a larger pressure drop appeared when the grease was delivered by pipes. Moreover, lubricating greases manifest excellent viscosity–temperature characteristics and thixotropic properties.5,6 The fluidity of lubricating greases increases with the increase of temperature and the fluidity can be fully recovered when the temperature drops to room temperature. Therefore, the delivery resistance can be decreased through heating the grease, and thus can improve the pumpability in the centralized lubricating system.However, oxidative and thermal degradation proceed when the lubricating grease was subjected to high temperatures, which would shorten the grease lubricating life.7 Cann et al.8,9 detected that the grease degradation initiated at the motion state of bearings and the temperature are important reasons for the degradation increase. Yu et al.10 found that the poor lubrication, which results in the thermal degradation of lubricating grease due to a high service temperature, is one of the most important reasons for serious local wear in fatigue failure analysis of roller bearings. Gonçalves et al.11 found that the thickener matrix structure varied after thermal ageing.
Therefore, the fluidity and pumpability of lithium lubricating grease would be improved through heating the grease, which is a highly possible result in the oxidative and thermal degradation of lubricating grease. The lubrication performance of lubricating grease is weakened due to the oxidative and thermal degradation. Wu et al.12 found that hydroperoxides were formed during the initial oxidation process, which could deteriorate the lubrication performance of di-2-ethylhexyl sebacate (DEHS). Kreivaitis et al.13 investigated the effect of thermal oxidation on the tribological properties of rapeseed oil and detected that the lubrication performance decreases with the process of oxidation due to structural changes in the oil. According to the research of Haseeb et al.,14 the biodiesel oxidizes with an increase of test temperature. In addition, the content of free water increases, and the antifriction and antiwear properties are improved as well. Furthermore, tribological properties of lubricating grease are generally used to evaluate its lubrication performance.15,16 Therefore, the variation of grease lubrication performance caused by heat treatment could be described via its tribological properties.
The objective of the present investigation was to provide the effect of heat treatment on the lubricating properties of NLGI 3 lithium lubricating grease. In this investigation, 120 °C, which is the highest temperature in standard ball bearing tests,11,17,18 was selected as the experimental temperature. The microstructure and infrared spectra of the lithium lubricating grease were investigated by FESEM and FTIR techniques, respectively, and the detailed variations are discussed. The strength of the microstructural network was estimated through rheological properties by using a rotational rheometer. The tribological properties were measured by a four-ball testing machine, and the action mechanisms are discussed as well. The research results could detect the effect of heat treatment on the lithium lubricating grease and provide applicable parameters for pumping NLGI 3 lithium lubricating grease.
2. Experiments
2.1 Materials
The NLGI 3 lithium lubricating grease sample was produced by Sinopec Lubricating Oil Co., LTD (China). The main components and technical data of the lubricating grease samples are listed in Table 1.| Thickener type | Thickener% (w/w) | Base fluid | Lubricating oil viscosity at 40 °C ASTM D-445 (mm2 s−1) | Dropping point ASTM D-566 (°C) | Consistency NLGI grade | Worked penetration ASTM D-217 (dmm) |
|---|---|---|---|---|---|---|
| Lithium 12-hydroxystearate | 12.9 | Mineral | 100 | 200 | 3 | 232 |
2.2 Sample preparation
The fresh grease with a weight of 10 g was spread in 250 mL glass beakers (thickness was about 1–2 mm) for four groups and they were kept at atmospheric pressure at 120 °C for 2 h, 4 h, 8 h and 24 h respectively by using a drying oven. At the same time, the mass loss of each sample was recorded. In order to limit the influence of water vapor on the weight loss experiment in this paper, the samples were weighed several times and the final weight was obtained when the weight of samples did not add up any longer. Besides, there are four groups of fresh grease, each of which has one glass tube with 2 g grease spread evenly. After heat treatment for 2 h, 4 h, 8 h and 24 h, the color of each sample was recorded.2.3 Property tests
The mass loss rate after heat treatment was computed according to the following formula:
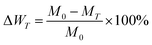 | (1) |
The microstructural characterisation of lubricating greases was carried out by means of FESEM (Supera 55 from Zeiss, Germany) at 10 kV. Grease samples were prepared by extracting the oil with n-heptane in several batches. Afterwards, the sample was dried at room temperature. Finally, the samples were coated with gold.
FTIR measurements were carried out in a VERTEX 80v FTIR spectrometer (Bruker, Germany) in transmission mode. Greases were placed on a silicon support in order to realize the infrared spectra.
Rheological measurements were performed under controlled stress and in a controlled shear rheometer (Physica MCR302 from Anton Paar, Germany) at a temperature of 25 °C by using plate–plate geometry (50 mm diameter, 1 mm gap). Small-amplitude oscillatory shear tests were carried out in a frequency range between 10−1 s−1 and 102 s−1. Apparent viscosity tests were performed at a constant shear rate (100 s−1) for 1000 s.
A four-ball testing machine with a speed of 1450 rpm was used (MMW-1 from Jinnan testing machine plant, China). The antifriction and antiwear tests were conducted under 300 N for 30 min. The maximum non-seizure load (PB value) was determined following the GB 12583-1998 standard, which is similar to ASTM D2783. A ball of 12.7 mm diameter made of GCr 15 with a 61–64 HRC was used. Each test was repeated three times to minimize data scattering. At the end of each test, the three lower balls were cleaned in petroleum ether and acetone respectively. These cleaned balls were available for wear scar diameter (WSD) measurement. The mean WSD on the three lower balls was measured with a light microscope to an accurate reading of 0.01 mm. Then the arithmetical average WSD of the three identical tests was calculated as the WSD.
3. Results and discussion
3.1 Mass loss rate and color
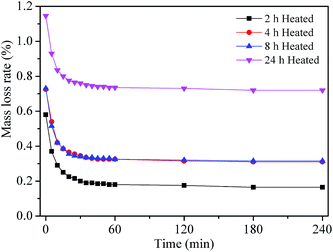
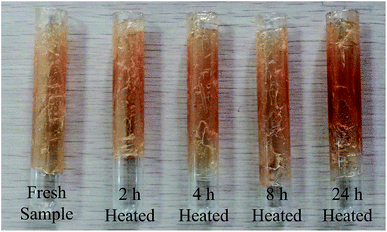
Fig. 1 shows the variation of the mass loss rate for grease samples as a function of placed time after heat treatment at 120 °C for 2 h, 4 h, 8 h and 24 h. It is found that the mass loss rate decreases along with the prolongation of placed time, and tends to be a stable value at last. This phenomenon is mainly attributed to the hydrophilic groups in the lithium lubricating grease. When the fresh grease samples were placed in a drying oven at 120 °C, the moisture combined with hydrophilic groups would be evaporated. However, the hydrophilic groups would absorb the moisture quickly if the samples were placed again in the air. Therefore, the final stable value should be used to investigate the mass loss rate, which could keep both evaporated and absorbed moisture balance and eliminate the effect of the hydrophilic groups. As can be observed in Fig. 1, the mass loss rate generally increases with the increase of heat treatment time. However, the mass loss rate would basically be the same during 4 h to 8 h, and increases obviously during 8 h to 24 h.The color variation, which may be owed to the formation of oil separation or even oxidation products, is one of the most obvious signs of the variation of the grease disperse system and component. Fig. 2 displays the optical photographs of a fresh grease sample and the heated grease samples with different test durations. It can be clearly seen that the color of the samples becomes slightly deeper with the increase of heat treatment time. There is no significant variation of sample color after heat treatment for 0 h to 4 h, while the color displays a slight variation after heat treatment for 8 h and 24 h. Previous studies17,18 have detected that the lubricating grease displays an obvious thermal ageing phenomenon with the formation of thermal ageing products after heat treatment for a long time, which needs sufficient time (10 days,17 288 hours (ref. 18)). In this work, the heat treatment time is much less than the time of thermal ageing. Therefore, the color variation may be attributed to oil separation of the lubricating grease because the base oil displays a deeper color than the fresh lubricating grease. When the oil separation appears and the base oil is unable to disperse again, the lubricating grease may display a deeper color. According to this extrapolation, it can be concluded that the oil separation increases along with the prolongation of heat treatment time for 0 h to 24 h. The results determine that the disperse system of lubricating grease has appeared with a visible variation, and the unique lubricating performance precisely connects with its disperse system. However, the accuracy of this extrapolation needs to be verified by experiments. Therefore, this variation should be investigated via the view of chemical and physical properties, and the lubricating performance could be further evaluated by its tribological properties.
3.2 Microstructure and FT-IR
Fig. 3 shows the microstructure of the experimental samples observed by FESEM, which were obtained at different heat treatment times. All experimental samples display a similar microstructure with a highly entangled fibrous structure. It can be clearly seen that the microstructure of the fresh grease sample displays a very clear and highly entangled fibrous structure, as shown in Fig. 3a. However, the entangled conditions of the 2 h to 24 h heated samples display less agglomerated and at a lower degree, as shown in Fig. 3b–e. And the variation of the fibrous structure becomes obvious with the increase of heat treatment time. The results explain that the physical entanglement of NLGI 3 lithium lubricating grease varied when the fresh samples undergo a short of heat treatment.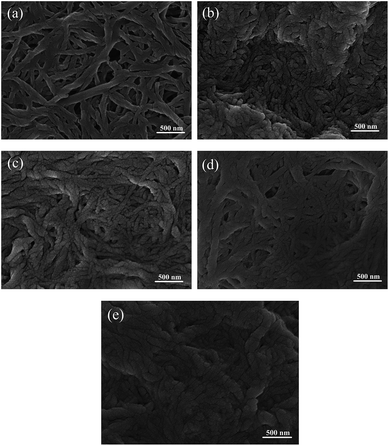 | ||
| Fig. 3 Microstructure of all grease samples (a) fresh sample; (b) 2 h heated; (c) 4 h heated; (d) 8 h heated; (e) 24 h heated. | ||
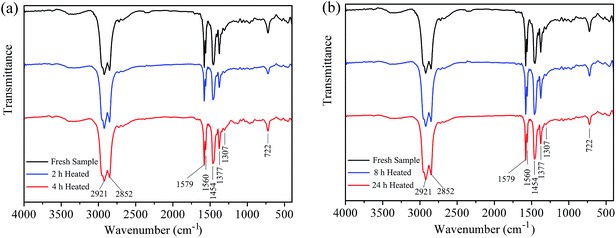
Fig. 4a and b display the FTIR spectra of the fresh grease sample and the heated grease samples at 120 °C for 2 h, 4 h, 8 h and 24 h. It can be clearly seen that there are several intense peaks for all the grease samples. The representatives of the characteristic peaks are the specific functional groups and bonds, as described in Table 2.
| Characteristic peak/cm−1 | Functional group assignment | Band | Source |
|---|---|---|---|
| 2921 | Asymmetrical stretching vibration of CH2 group | C–H | |
| 2852 | Symmetrical stretching vibration of CH2 group | C–H | |
| 1579 | Asymmetrical stretching vibration of COO group | COO | Thickener |
| 1560 | Symmetrical stretching vibration of COO group | COO | Thickener |
| 1454 | Asymmetrical deformation vibration of CH3 group | C–H | Base oil |
| 1377 | Bending vibrations of CH2 groups | C–H | Base oil |
| 1307 | Twisting vibration of CH2 group | C–H | |
| 722 | Overlapping of the CH2 rocking vibration and the out-of-plane vibration of cis-disubstituted olefins | C–H |
As can be observed, the infrared spectra of the heated grease samples for 2 h to 24 h are basically in accordance with the infrared spectra of the fresh grease sample, and no new intense characteristic peaks appear in the whole range of wavenumbers.
Previous studies17,18 have detected that the new peaks at 1715 cm−1 and 1736 cm−1, characteristic of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (ketones and esters, respectively) formed by oxidation of the sample, will appear for the aged grease samples when the grease sample undergoes the thermal ageing phenomenon. Meanwhile, the peaks of 1560 cm−1 and 1579 cm−1, characteristic of the COO bond which comes from the composition of the thickener, will become weakened. Moreover, the peak of 1377 cm−1, characteristic of a C–H bond which comes from the CH2 group of the base oil, will be strengthened due to the breakage of a long molecular chain.
O bond (ketones and esters, respectively) formed by oxidation of the sample, will appear for the aged grease samples when the grease sample undergoes the thermal ageing phenomenon. Meanwhile, the peaks of 1560 cm−1 and 1579 cm−1, characteristic of the COO bond which comes from the composition of the thickener, will become weakened. Moreover, the peak of 1377 cm−1, characteristic of a C–H bond which comes from the CH2 group of the base oil, will be strengthened due to the breakage of a long molecular chain.
As can be observed in Fig. 4a and b, the new peaks at 1715 cm−1 and 1736 cm−1 do not arise in the FTIR spectra. Therefore, the aged products (ketones and esters) would not be released from the grease samples after heat treatment for 2 h to 24 h.
The FTIR results indicate that thermal degradation of the grease samples does not appear after heat treatment at 120 °C for 2 h to 24 h. In other words, no obvious chemical structure variation appears at above operation conditions. However, according to the microstructure results, it can be noticed that highly entangled and agglomerated fibres are apparent for the fresh grease sample, whilst a lower density and less agglomerated fibres are apparent after heat treatment. Therefore, the color of the samples becomes slightly deeper along with the increase of heat treatment time, which is attributed to a higher oil separation degree of the grease samples due to the variation of physical entanglement.
3.3 Rheological properties
Rheological properties are major factors that determine the strength of the three-dimensional network of lubricating greases. Therefore, rheological properties can usually be used to evaluate the role of lubricating greases in the lubrication of moving parts.19 The storage (G′) and loss (G′′) modulus on a frequency sweep test within the linear viscoelasticity range are used to estimate the strength of the network structure. The viscosity of constant shear rate can be used to simulate the variation of viscosity in the lubrication process of stable revolving moving parts, where the lubricating grease approximatively suffers constant shear.In addition, the lubricating grease has excellent viscosity–temperature characteristics, and detailed discussion has been carried out in many references.5,6 In other words, the rheological property factors are not unique for a given system but are a function of temperature. In order to evaluate the effect of heat treatment time on the rheological properties, the experimental temperature was selected at 25 °C.
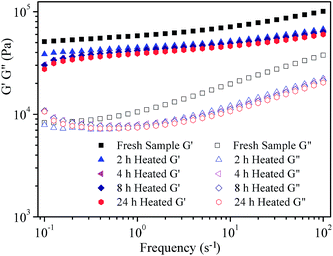
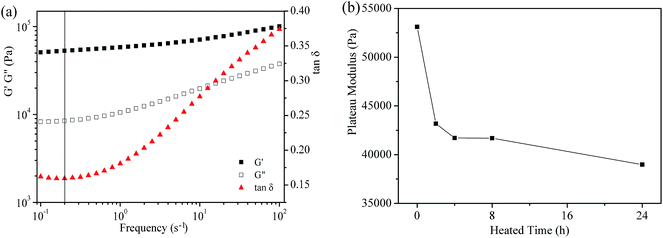
Fig. 5 shows the variation of the storage (G′) and loss (G′′) modulus on frequency within the linear viscoelasticity range for all grease samples as a function of heat treatment time. It can be observed that the values of the storage modulus (G′) are always higher than those of the loss modulus (G′′) in the frequency range between 10−1 s−1 and 102 s−1. In addition, the values of both the storage modulus (G′) and the loss (G′′) modulus decrease with the increase of heat treatment time. These results reveal the idea that both stored energy in an elastic deformation process and lost energy in a viscous flow process decreases when the fresh grease samples undergo a short heat treatment. In other words, the fluidity of the lubricating grease is improved after heat treatment. This is mainly because the physical entanglements of lithium lubricating grease became less agglomerated and at a lower degree after heat treatment.
Combined with the study of Martín-Alfonso et al.,19 the plateau modulus (G0N) is usually considered as a measure of the aggregation number among the dispersed structural units. This characteristic parameter is related to the strength of the microstructural network. Therefore, the plateau modulus (G0N) can be used as a reference parameter to evaluate the variation of viscoelastic behavior of the experimental samples as a function of the heat treatment time. Moreover, the plateau modulus (G0N) can be easily obtained through a straightforward method from the frequency sweep data, as shown in eqn (2).
G0N = [G′]tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ→minimum δ→minimum
| (2) |
As can be observed in Fig. 6a, the plateau modulus (G0N) of the fresh grease sample at 25 °C is obtained according to eqn (2). Similarly, the plateau modulus (G0N) of the other grease samples is given in Fig. 6b. It can be found that the values of the plateau modulus (G0N) show an overall decreasing tendency and the data could be divided into three parts: obviously decreased (0–4 h), stable value (4–8 h) and slightly decreased (8–24 h). The results indicate that the entanglement strength of the grease microstructural network is evidently weakened after heat treatment for a short time.
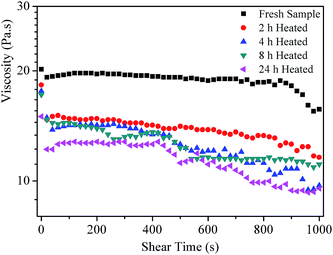
Fig. 7 displays the variation of viscosity of all the grease samples at a constant shear rate as a function of shear time. It can be clearly seen that the viscosity of the grease samples decreases with the increase of heat treatment time. The results support the idea further that the fluidity of the lubricating grease samples is obviously improved after heat treatment for a short time.
Combined with the results of both the FESEM and FTIR analysis, the variations of the plateau modulus (G0N) and viscosity are caused by the variation of physical entanglements rather than products of chemical variation. In other words, a short heat treatment can merely vary the physical entanglements and is not enough to alter the chemical structure.
However, the specific lubrication properties of the lubricating grease are generally closely related to the physical entanglements.20 Therefore, the effect of heat treatment on the lubricating properties of lubricating grease via the variation of physical entanglements should be studied by the tribological properties.
3.4 Tribological properties
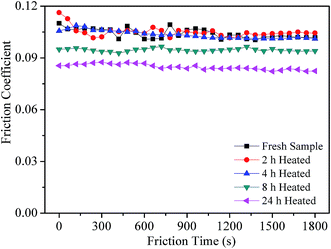
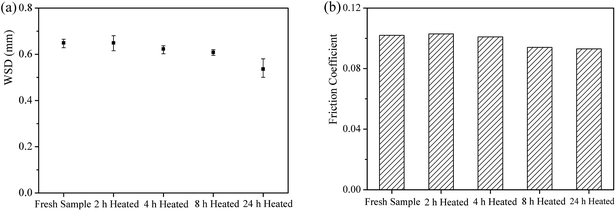
Fig. 8 shows the friction coefficient of all the grease samples as a function of friction time. It can be seen that the variation of the friction coefficient could be divided into two parts according to the heat treatment time. From 0 h to 4 h, there is no obvious regularity of the friction coefficient. However, the samples after heat treatment for 8 h and 24 h exhibit lower friction coefficients with the increase of heat treatment time.Fig. 9a and b display the WSD and the stable friction coefficient of all the grease samples as a function of the heat treatment time. It can be seen that the variations of both WSD and the friction coefficient display good correspondence. In other words, as the friction coefficient decreases with the increase of heat treatment time, the value of WSD decreases. This character is mainly attributed to the unchanged component of all the experimental samples, as the detailed result has been discussed in Section 3.2.
Fig. 10 shows the morphologies of the worn surfaces lubricated by different grease samples. It can be clearly seen that the worn surfaces of the steel balls lubricated by different grease samples are very similar. There are no obvious differences among wear scars under different lubrication conditions. It can be concluded that all the samples display a similar mechanism of antifriction and antiwear properties. When the friction coefficient decreases with the increase of heat treatment time, the lubrication condition of the rubbing surfaces could be improved and the value of WSD will decrease. Therefore, the antifriction and antiwear properties of the lithium lubricating grease are improved with the increase of heat treatment time.
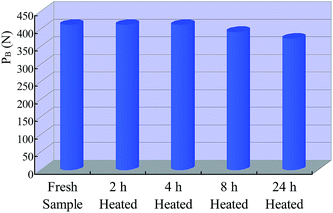
Fig. 11 shows the extreme pressure load (PB) of all the grease samples. As a comparison, the PB value of the fresh lithium lubricating grease is also given. It can be seen that the value of PB keeps is stable after heat treatment for 0 h to 4 h and declines slightly with the increase of heat treatment time from 4 h to 24 h. The results explain that the extreme pressure property, which is the load-carrying capacity of lubricating film, will be weakened when the lithium lubricating grease undergoes heat treatment, and it simultaneously decreases with the increase of heat treatment time.
3.5 Lubrication mechanism analysis and discussion
The results of the tribological properties show that the antifriction and antiwear properties have been improved with the increase of heat treatment time, while the extreme pressure property gradually decreases. Meanwhile, the FESEM and FTIR results show that the chemical composition has not been changed during heat treatment, while the strength of the grease fibrous structure displays a tendency to decrease. In other words, the lubrication performances of the lithium lubricating grease vary by short heat treatments, while it is not caused by the variation of its chemical composition but by its physical entanglement strength.Several researchers found that some polar groups (alcohols, acids and monoesters) could significantly improve the lubrication performance of oil lubrication by formation of stable boundary adsorption films on the rubbing surfaces.12–14,21,22 In this paper, the above results have excluded the possibility of a polar group effect. According to the results of the FTIR spectra, there is no obvious variation of all the heated samples. Especially, no new functional group appeared on the basis of the characteristic peak analysis. Therefore, the variation of lubricating properties is mainly caused by the variation of physical entanglement.
The replenished mechanism of grease lubrication is entirely different from that of oil lubrication. For oil lubrication, the lubricant of the rubbing surface could be replenished easily with oil for better fluidity. However, lubricating greases manifest weak fluidity, especially NLGI 2, 3 lubricating greases. Lubricating grease is easily lost from the rubbing surface (the track of rolling bearings) either due to fluid displacement in the inlet region or as squeeze flow from the contact.23–25 The lubricant replenishment mechanism of the contact zone during grease lubrication was given by Cann.26 The lubrication process of grease in the track of rolling bearings could be divided into three stages: the initial stage, layer formation stage and stabilized stage. In the initial stage, the grease undergoes continuous shear from the inlet to contact zone. The majority of grease is rapidly pushed away from the track of rolling bearings. After this stage, only a fraction of the grease deposits in the track and forms a grease layer. The structure of grease is broken down and free oil is squeezed from the contact with continued rolling. In the stabilized stage, the grease of the side of the contact zone gradually releases base oil due to its broken down structure. The base oil or grease reflows into the contact to replenish the lubricant of the rubbing surface. Therefore, efficient replenishment of grease lubrication plays an important role in keeping favorable lubrication of the rubbing surface for a long time.
The results of the friction and wear experiments showed that the antifriction and antiwear properties have been improved with increased heat treatment time. This mainly attributes to the minor non-chemical variation of grease during heat treatment for a short time. The mass loss rate and color contrast experimental results show that the lithium lubricating grease samples progressively lose weight and release base oils, which indicates that the lithium lubricating grease samples vary during heat treatment. The structural strength of soap fiber is weakened after heat treatment, and this has been confirmed by rheological property experiments. Besides, the viscosity of the lithium lubricating grease at a constant shear rate decreases with the increase of heat treatment time, and the grease displays better fluidity on the side of the contact. The base oil is more easily released from the soap fibers of the grease. In combination with the above factors, the lubricant of the rubbing surface could more easily be replenished with oil or grease reflow from the side of the contact in the friction and wear experiments, which could improve the lubrication performance of the heat treated grease by formation of a stable replenishment circulation on the rubbing surfaces.
Combining the above results, it can be found that the lubricating properties of NLGI 3 lithium lubricating grease has not varied after 0–4 h heat treatment and the grease displays a tiny non-chemical variation after 8 h and 24 h heat treatment. Therefore, the heat treatment time should been controlled within 4 h or less and at 120 °C to ensure the impervious lubricating property of NLGI 3 lithium lubricating grease when the heat treatment is used to improve its pumpability.
4. Conclusions
According to the above results and discussions, the following conclusions can be obtained:(1) The mass loss rate and color contrast analysis showed that the lithium lubricating grease samples progressively lose weight and release base oil. Visible variation of the lithium lubricating grease samples appear during heat treatment at 120 °C for 2 h to 24 h, especially from 8 h to 24 h.
(2) The FTIR spectra of all the heated samples have not changed relatively to the fresh grease sample. Especially, no new functional group appeared on the basis of the characteristic peak analysis. However, the high entanglement network microstructure became less agglomerated and at a lower degree after short heat treatment times, and the strength of the fibrous structure decreases with the increase of heat treatment time.
(3) The oil separation is facilitated and the viscosity at a constant shear rate decreases after heat treatment at 120 °C for 2 h to 24 h. These performances realize efficient replenishment of the base oil or grease for the contact zone. The antifriction and antiwear properties have been improved by the formation of better oil reflow on the contact zone, which are inconspicuous during 0 h to 4 h. However, the extreme pressure property gradually decreases with the increase of heat treatment time.
(4) The heat treatment time should been controlled within 4 h or less and at 120 °C to ensure the impervious lubrication property of NLGI 3 lithium lubricating grease when the enhancing temperature technique is used to improve its pumpability.
Acknowledgements
This work is supported by the National Natural Science Foundation (51006117), the Graduate Student Innovation Training Project in Jiangsu Province (KYLX_1372) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- M. Paszkowski, S. Olsztyńska-Janus and I. Wilk, Tribol. Lett., 2014, 56, 107–117 CrossRef CAS
.
- M. Paszkowski, Proc. Inst. Mech. Eng., Part J, 2012, 227, 209–219 CrossRef PubMed
.
- J. X. Li, E. Höglund, L. G. Westerberg, T. M. Green, T. S. Lundström, P. M. Lugt and P. Baart, Tribol. Int., 2012, 54, 94–99 CrossRef PubMed
.
- M. A. Delgado, J. M. Franco, P. C. Partal and C. Gallegos, Chem. Eng. Process., 2005, 44, 805–817 CrossRef CAS PubMed
.
- M. C. Sánchez, J. M. Franco, C. Valencia, C. Gallegos, F. Urquiola and R. Urchegui, Tribol. Lett., 2011, 41, 463–470 CrossRef PubMed
.
- M. A. Delgado, C. Valencia, M. C. Sánchez, J. M. Franco and C. Gallegos, Tribol. Lett., 2006, 23, 47–54 CrossRef CAS
.
- S. Hurley, P. M. Cann and H. A. Spikes, Tribol. Trans., 2000, 43, 221–228 CrossRef CAS PubMed
.
- P. M. Cann, Tribol. Int., 2006, 39, 1698–1706 CrossRef CAS PubMed
.
- P. M. Cann, M. N. Webster, J. P. Doner, V. Wikstrom and P. Lugt, Tribol. Trans., 2007, 50, 187–197 CrossRef CAS PubMed
.
- Z. Q. Yu and Z. G. Yang, J. Fail. Anal. Prev., 2011, 11, 158–166 CrossRef
.
- D. Gonçalves, B. Graça, A. V. Campos, J. Seabra, J. Leckner and R. Westbroek, Tribol. Int., 2015, 87, 160–170 CrossRef PubMed
.
- Y. X. Wu, W. M. Li, M. Zhang and X. B. Wang, Tribol. Int., 2013, 64, 16–23 CrossRef CAS PubMed
.
- R. Kreivaitis, J. Padgurskas, M. Gumbytė, V. Makarevičienė and B. Spruogis, Transport, 2011, 26, 121–127 CrossRef
.
- A. Haseeb, S. Y. Sia, M. A. Fazal and H. H. Masjuki, Energy, 2010, 35, 1460–1464 CrossRef CAS PubMed
.
- X. H. Wu, Q. Zhao, M. Zhang, W. M. Liu, G. Q. Zhao and X. B. Wang, RSC Adv., 2014, 4, 54760–54768 RSC
.
- M. Taher, F. U. Shah, A. Filippov, P. Baets, S. Glavatskih and O. N. Antzutkin, RSC Adv., 2014, 4, 30617–30623 RSC
.
- I. Couronne and P. Vergne, Tribol. Trans., 2000, 43, 788–794 CrossRef CAS PubMed
.
- T. J. Shen, M. H. Hu, R. G. Liu and Q. L. Liu, Tribology, 2011, 31, 581–586 CAS
.
- J. E. Martín-Alfonso, C. Valencia, M. C. Sánchez and J. M. Franco, Tribol. Trans., 2012, 55, 518–528 CrossRef PubMed
.
- J. E. Martín-Alfonso, A. Romero, C. Valencia and J. M. Franco, J. Ind. Eng. Chem., 2013, 19, 580–588 CrossRef PubMed
.
- N. J. Fox and G. W. Stachowiak, Tribol. Int., 2007, 40, 1035–1046 CrossRef CAS PubMed
.
- N. J. Fox and G. W. Stachowiak, Lubr. Eng., 2003, 59, 15–20 CAS
.
- P. M. Cann and A. A. Lubrecht, Tribol. Ser., 2003, 43, 745–750 CrossRef
.
- P. M. Cann, B. Damiens and A. A. Lubrech, Tribol. Int., 2004, 37, 859–864 CrossRef CAS PubMed
.
- L. Huang, D. Guo and S. Z. Wen, Tribol. Lett., 2014, 55, 483–492 CrossRef CAS
.
- P. M. Cann, Tribol. Trans., 1996, 39, 698–704 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |