Mussel inspired functionalization of carbon nanotubes for heavy metal ion removal†
Abstract
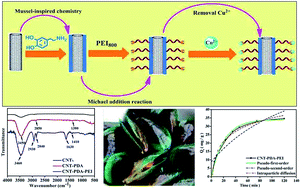
Carbon nanotubes (CNTs) have been widely used as adsorbents to remove various environmental pollutants because of their unique one dimensional structure, large surface areas and amount of micropores. However, the adsorption capacity of unmodified CNTs toward heavy metal ions is still limited due to their poor dispersibility and lack of functional groups. In this work, a novel strategy has been developed to prepare polyethylenimine functionalized CNTs via the combination of mussel inspired chemistry and the Michael addition reaction. The successful preparation of CNTs with amine groups was confirmed by a series of characterization measurements such as transmission electron microscopy, Fourier transform infrared spectroscopy, and thermal gravimetric analysis. Furthermore, the adsorption application of these amine functionalized CNTs toward Cu2+ was also examined. The effects of various factors including contact time, pH values, temperature and initial Cu2+ concentrations on the adsorption capability of the amine functionalized CNTs were also investigated. Langmuir and Freundlich models were used for thermomechanical analysis. The pseudo-first-order, pseudo-second-order and intra-particle diffusion models were used for the kinetics analysis. The results demonstrated that CNTs can be successfully functionalized with amine groups through a rather facile and mild bioinspired strategy. These amine functionalized CNTs exhibited a much enhanced adsorption efficiency toward Cu2+. Given the strong and versatile adhesion of PDA to various materials, the bioinspired strategy described in this work could also be utilized for the fabrication of many other nanocomposites for environmental applications.

 Please wait while we load your content...
Please wait while we load your content...