PVDF layer as a separator on the solution-side of air-cathodes: the electricity generation, fouling and regeneration†
Abstract
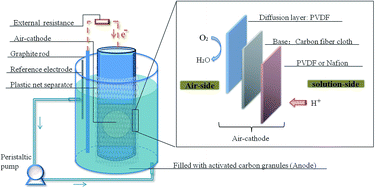
Cost effective air-cathodes are very important for application of Microbial Fuel Cells (MFCs). Carbon fiber cloth was used as the base for preparing air-cathodes with cheap polyvinylidene fluoride (PVDF) coating on the solution-side to replace expensive Nafion as a separator regardless of the ion exchange part. The separator on the solution-side was a vital part for stable electricity generation. Another PVDF layer was coated on the air-side via a thickness-controllable method as the diffusion layer. The comparisons between Nafion and PVDF on the solution-side of cathode in electricity generation and fouling/regeneration were tested in MFCs without any catalyst. The cell voltage of the Nafion air-cathode MFC (0.23 V) was slightly higher than the PVDF MFC (0.2 V). Biofouling and cation deposition on air-cathodes became more severe with time, negatively affecting electricity generation. Soaking the air-cathode with solution-side Nafion in HCl (maximum power density: 338.1 mW m−2) can regenerate the cathodes and enhance the electricity generation much more than that regenerated by ultraviolet light (UV) irradiation.

 Please wait while we load your content...
Please wait while we load your content...