Selective detection of fluoride using fused quinoline systems: effect of pyrrole†
Abstract
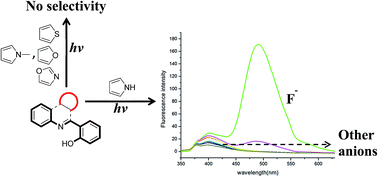
Selective detection of fluoride by 2-(3H-pyrrolo[2,3-c]quinolin-4-yl)phenol is reported using a turn-on fluorescence method. The presence of a pyrrole ring along with substituted o-phenol is essential as their absence results in either loss of selectivity or decrease in fluorescence intensity.

 Please wait while we load your content...
Please wait while we load your content...