Fe3C and Mn doped Fe3C nanoparticles: synthesis, morphology and magnetic properties†
Xiaobai Wang,
Peng Zhang,
Wei Wang,
Xiang Lei and
Hua Yang*
College of Chemistry, Jilin University, Changchun, 130012, PR China. E-mail: huayang86@sina.com
First published on 17th June 2015
Abstract
Fe3C and Mn doped Fe3C nanoparticles (NPs) were prepared by a sol–gel method. The structures, morphology and magnetic properties are researched. From Transmission Electron Microscopy (TEM) results, it is shown that the morphology of the Fe3C and Mn doped Fe3C NPs are different. From vibrating sample magnetometer (VSM) results, it is indicated that their special saturation magnetization (Ms) values tend to decrease with increasing the Mn doping concentration, which can be correlated with changes in the lattice constant due to the Mn ion incorporation in Fe3C.
Introduction
Iron carbide materials have become prominent in various domains of scientific research and applications.1–7 Primarily, iron carbides consist of carbon atoms occupying the interstices between close-packed iron atoms and the presence of carbon atoms provides iron carbides with excellent properties compared with other oxide materials.8 For example, iron carbides possess a maximum bulk magnetization of 140 emu g−1 which is 52% larger than the value of 92 emu g−1 for iron oxide.9 In addition, iron carbides exhibit the elevated air-stability10 and low toxicity.11 Such properties make the iron carbides NPs can find applications in the field of catalysis,12–14 electrochemistry15,16 bioimaging17,18 and magnetic storage.19Despite their numerous advantages, iron carbide NPs have been much less investigated than iron oxide or other oxide materials, primarily because there are still big challenges in the synthetic strategy of iron carbide NPs. Recently, Hu and co-workers fabricated a few-layer graphene sheets with encapsulated Fe3C nanoparticles from pyrolysis of volatile non-graphitic precursors.20 Liao et al. prepared graphitic carbon/Fe3C nanocomposites by a solid-state pyrolysis approach.21 Daniel et al. synthesized Fe3C/C composite nanoparticles via a thermal decomposition of the ferrocene-swollen template polystyrene particles.22 However, the accompanying complicated procedures in current synthetic routes produce iron carbide NPs without control over the size and morphology, which is highly necessary for practical applications.
On the other hand, researchers recently found that the substitution with various metal ions into magnetic NPs host can introduce a high catalytic activity, high Curie temperature (TC), high coercivity, or moderate saturation magnetization.2,23–25 For example, recently, Ji et al. prepared highly activated K-doped iron carbide nanocatalysts via a Multi-step method.2 Ranjith et al. prepared co-doped CeO2 NPs by a low-temperature co-precipitation method, and the NPs showed relative a high coercivity value at room temperature.26 Researchers have studied the electronic structure and formation enthalpy of (Fe, M)3C (M = Cr/Mn/Co/Ni). They concluded that Cr/Mn doping can enhance the stability of Fe3C, while Co/Ni doping is opposite.27,28 Gao et al. also investigated the surface structure and the stabilities of orthorhombic Fe3C and Fe2CrC with DFT. They concluded that the surface stability of both Fe3C and Fe2CrC gradually decreases from (001) and (010) to (100), and the surface of Fe2CrC is less stable than that of Fe3C.29 However, the influence of magnetic transition metal doping on Fe3C is still limited to the theoretical study. Thus it is our interest to take the next step in the direction of simple, green, and low-cost approach for the synthesis of transition metal doped Fe3C NPs. In addition, to the best of our knowledge, the synthesis of Mn doped Fe3C NPs has been rarely reported.
In this study, we demonstrate the use of melamine, an important carburization reagent,30 for the synthesis of Fe3C and Mn doped Fe3C NPs. During the course of the experiment, CTAB is involved. When CTAB is mixed with a solution of melamine heated in a water bath, it will form a gel and will disperse an aqueous iron precursor within this gel. When heating in horizontal electric tube furnace, the melamine is decomposed to form various intermediates. Then, the intermediates act as carbon source to react with Fe and Fe3O4 to generate target products. The morphology and magnetic properties of Fe3C and Mn doped Fe3C NPs are investigated. Moreover, the different doping concentrations of Mn make the Fe3C NPs display distinct magnetic properties.
Experimental
Synthesis of precursors
FeCl3·6H2O, MnCl2·4H2O, CTAB and melamine were obtained from commercial sources and used as received without further purification. FeCl3·6H2O (1.35 g), CTAB (2.0 g) and the melamine (1.5 g) were dissolved in a solution of distilled water (40 mL) and ethanol (40 mL) in a small beaker, the certain amount of MnCl2·4H2O (0–0.4 g) was also involved in this step. The beaker was transferred to an ultrasound water bath at 80 °C for 3 h and stirred during the course. Then the above mixture was dried at 80 °C in an oven under air atmosphere. Finally, the orange precursors were collected from the beaker and ground up into a fine powder using an agate mortar and pestle. The synthesis of Mn doped Fe3C NPs was carried out under atmospheric pressure.Synthesis of Mn doped Fe3C NPs
A certain amount of the above prepared precursor was loaded into an alumina boat. The boat was put into the center of a quartz glass tube. The tube was placed into a horizontal electric tube furnace. A thermocouple was inserted into the center of the tube and kept very near to the boat to measure its exact temperature. High-purity nitrogen (purity ≥ 99.9%) was introduced into the tube at a controlled flow rate. Then the furnace was heated at 10 °C min−1 to 650 °C, kept for 3 h and cooled to room temperature naturally. Finally, the black powders were collected from the alumina boat and ground up into a fine powder. The preparation process is illustrated in Scheme 1.Characterization
X-ray diffraction (XRD) patterns were recorded to analyze the phase and crystal structure of the as-synthesized samples using a Shimadzu XRD-6100, operated at a step size of 0.06° in the range of 20–80° and with Cu Kα radiation (λ = 0.15405 nm). The samples were examined to obtain size/shape images of nanostructured materials by and Philips Tecnai G2 F20 high resolution transmission electron microscopy (HRTEM). Firstly, they were dispersed in absolute ethyl alcohol by sonification for 15 min. Then drops of the black suspension were deposited on a holey carbon-coated copper grid and ethanol was allowed to evaporate in air. Raman spectrum was measured with a JobinYvon/HORIBALabRam ARAMIS Raman spectrometer with the radiation from an air-cooled He Ne laser (633 nm). Thermogravimetric Analysis (TGA) measurement was carried out using a DTG-60 (Shimadzu). The magnetic properties of the as-synthesized samples were tested using a vibrating sample magnetometer (VSM, LakeShore 7404). The maximum magnetic field is 17 000 Oe.Results and discussion
Structures and morphology of Fe3C and Mn doped Fe3C NPs
 | ||
| Fig. 1 (a) XRD patterns of Fe3C and Mn doped Fe3C NPs. (b) The graph of the crystal size and lattice constant vs. Mn doping concentration. | ||
From the strong and broad peaks in Fig. 1(a), it is indicated that the particle sizes are in the nanometre range. The XRD pattern of Fe3C shows diffraction peaks corresponding to (211), (102), (220), (031), (112) and (221) planes located at 2θ = 42.9, 43.76, 44.5, 44.9, 45.8 and 49.1° respectively. The peak at around 2θ = 26°, can be indexed to carbon (JCPDS card no. 41-1487). The intensity of the carbon peak is made weaker with increasing Mn-dopant. It is worth mentioning that the weak peak for Mn doped Fe3C at around 2θ = 53° can be ascribed to Mn (JCPDS card no. 88-2327), indicating the existence of trace amounts of Mn in the composites. Using the Debye Scherrer equation, the crystallite size of the Fe3C and Mn doped Fe3C NPs are estimated to be 35–43 nm based on the characteristic peak at 2θ = 44.9 corresponding to (031) crystal plane. The particle sizes increased with increasing the Mn-dopant, which may be due to the Fe being replaced with Mn atoms, which have a bigger radius than that of the Fe atoms in the samples. The lattice constant is calculated using the surface spacing formula and Bragg equation as follow:
| 1/d2 = h2/a2 + k2/b2 + l2/c2 | (1) |
2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = λ θ = λ
| (2) |
The calculated lattice parameter constant (a) for the Fe3C is 0.5085 nm and this value is consistent with that of the bulk Fe3C (0.5089 nm, JCPDS card no. 65-2413). Fig. S1† is the magnified XRD patterns of Fe3C and Mn doped Fe3C NPs (40–50°), it can be seen that the strong peak at 2θ = 44.9° becomes sharper with increasing the Mn-dopant. Moreover, comparing with the undoped one, all of the doped samples have no significant difference except the slight angles shift, implying probably the Mn substitution of Fe in the Fe3C lattice. In Fig. 1(b), it is shown that with increasing the doping concentration of Mn, the lattice parameter values of the Fe3C are increased slightly.
Fig. 2(b) shows the typical higher-magnification image of three particles. A typical higher-magnification image of a single particle is shown in Fig. 2(c), indicating that the particle has a core–shell structure and its particle size is about 47 nm. Fig. 2(d) shows the HRTEM image of the box region in Fig. 2(c). It is confirmed that the Fe3C nanoparticle is encapsulated by a shell with a thickness of about 8.4 nm. It can also be seen that the well-crystallized structures with lattice fringes of about 0.34 and 0.38 nm, corresponding to an interplanar spacing of graphite (002) and Fe3C (011) crystal plane, respectively. The formation of graphite shell may be due to the fact that the Fe3C NPs can play the role of catalyst to change the amorphous carbon into the graphite carbon. Liao et al. have also reported that the graphitic carbon appeared after the formation of Fe3C NPs.21 In the Raman spectrum Fig. 2(e), the G peak at 1600 cm−1 and the D peak at 1328 cm−1 suggest the presence of the graphitic carbon.31,32 This is evidence that there is graphitic carbon and amorphous carbon in the as-prepared samples. Meanwhile, the peaks at the low wave numbers of 216 and 277 cm−1 in Fig. 2(e) are ascribed to Fe3C NPs.
Fig. 3(a) shows that the 20% Mn doped Fe3C NPs are cube shaped.
It is worth mentioning that the morphology of Mn doped Fe3C NPs is drastically different from the Fe3C NPs. This should be due to the fact that the Mn-dopant atoms access into the Fe3C lattice and result in a distorted lattice of Fe3C as well as the effects on the morphology of Fe3C NPs. Meanwhile, there is no graphite carbon shell surrounding the Fe3C NPs, which indicates that the Mn-dopant has an adverse effect on the graphite carbon formation. In addition, the particle size of the particles is increased with increasing the Mn-dopant, which corresponds to the XRD analysis. This provides further evidence for the view for the replacement of Mn atoms in the crystal lattices. Fig. 3(b) shows a typical higher-magnification image of a 20% Mn doped nanoparticle and its size is about 89 nm. Fig. 3(c) shows the HRTEM image of the circle region in Fig. 3(a). The fringe spacing of 0.25 nm is observed in the HRTEM image, corresponding to the (200) crystal plane of Fe3C.33 The high angle annular dark field scanning TEM (HAADF-STEM, in Fig. 3(d)) reveals that there are carbon matrix and cubic Mn doped Fe3C NPs. Each of the components appear uniform from elemental mapping of carbon (red), Fe (blue), Mn (green) and oxygen (yellow) in Fig. 3(e)–(h). Carbon and oxygen are detected over the whole area, two important aspects should be responsible for the presence of oxygen: (1) even though the fresh samples are passivated by ethanol, slight surface oxidation of the Mn doped Fe3C NPs is inevitable due to atmospheric exposure during the sampling processes. (2) Another important reason is the surface adsorbed air and oxygenic functional groups on the samples. Mn is mainly found in the Fe-rich area. It is indicated that Fe atoms are replaced with Mn atoms in the samples. The presence of Mn, Fe and C elements in the samples are further confirmed by energy dispersive X-ray spectroscopy (EDXS) on individual NPs in Fig. 3(i). In addition, from Fig. S3,† it is shown that there are spherical shape and cube shape particles for 10% Mn doped Fe3C.
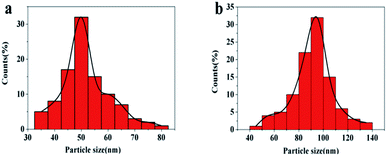
In order to learn more about the particle size distribution of Fe3C NPs and 20% Mn doped Fe3C NPs, we randomly investigated one hundred NPs using TEM images obtained from Fe3C and 20% Mn doped Fe3C NPs respectively. The distribution of the particle sizes in the samples is narrow in the range of 40–50 nm in Fig. 4(a). Moreover, it can also be seen that the average particle sizes of the 20% Mn doped Fe3C NPs are 80–90 nm in Fig. 4(b).
In order to quantify the amounts of carbon in Fe3C and Mn doped Fe3C NPs, TGA was performed on different samples (Fig. S2†). The weight reduction between 200 and 485 °C is ascribed to dehydration and the decomposition of functional groups adsorbed on the surface of carbon; weight reductions between 485 and 710 °C can be ascribed to the oxidation of carbon and Fe3C.3 The carbon is converted to CO2 gas, Fe3C and Mn doped Fe3C NPS are oxidized into Fe2O3 and MnO or MnO2. Thus for, it can be concluded that the carbon content for Fe3C, 10% Mn doped Fe3C and 20% Mn doped Fe3C are 58.8, 59.3 and 60.4% respectively. Indicating that with increasing the Mn-dopant, the amounts of carbon in each sample vary little.
Magnetic characterization
Fig. 5(a) shows the magnetization hysteresis loops for the Fe3C and Mn doped Fe3C NPs. | ||
| Fig. 5 (a) M–H plots of the Fe3C and Mn doped Fe3C NPs, (b) The graph of the Ms and Hc vs. doping concentration. | ||
For both the Fe3C and Mn doped Fe3C NPs, the ferromagnetism properties are displayed. It can be noticed that the Ms values tend to decrease with increasing the Mn doping concentration. In contrast, the coercivity (Hc) values are increased from 179 Oe to 225 Oe. From the inset picture in Fig. 5(a), it is demonstrated that the magnetic NPs can be easily recovered from suspensions by applying a magnetic field induced by a neodymium permanent magnet placed next to the vial. The Ms and Hc values vs. Mn doping concentration are summarized in Fig. 5(b). All the Ms values for Fe3C and Mn doped Fe3C NPs are smaller than the value of the bulk material, which is around 140 emu g−1. This decrease can be ascribed to both the carbon matrix surrounding the Fe3C and the limited magnetic moment lattice expansion. Based on the above data, we think that four important aspects should be responsible for the changes of the magnetic parameters: (1) the changes in the magnetic properties with the increase of Mn concentration can be correlated with changes in the lattice constant due to the Mn atoms incorporation in Fe3C. Any changes in the lattice constant will imply strong electronic bonding between Mn, Fe and C atoms. (2) In our previous paper,34 we concluded that the graphitization degree may be related closely to the magnetic parameters of the products. (3) The morphologies of Fe3C and Mn doped Fe3C samples are different, it can be deduced that the magnetic parameters may be affected by the morphology. (4) Because the ionic magnetic moment of Mn is less than that of Fe, the Ms values of Mn doped Fe3C NPs are also decreased.
Conclusions
Fe3C and Mn doped Fe3C NPs were synthesized via a simple sol–gel method. From the XRD patterns, it is shown that these samples are single phase and in the nanometre range. The shape of the Fe3C NPs is spherical with core–shell structures, but the shape of the Mn doped Fe3C NPs is cubic. The particle size of Mn doped Fe3C NPs is increased with increasing the Mn-dopant, which may be due to the replacement by Mn atoms in the samples. From the elemental mapping, it is shown that the Mn atoms are mainly found in the Fe-rich area, indicating that the doped Mn atoms well replaced the Fe atoms in the samples. From VSM, it is revealed that both the Fe3C and Mn doped Fe3C NPs displayed ferromagnetic properties. The Ms values tend to decrease with the increasing Mn doping concentration, which can be correlated with changes in the lattice constant due to the Mn atoms incorporation in Fe3C, and so on.Acknowledgements
This work was supported by the National Natural Science Foundation of China.Notes and references
- G. Zhang, S. Peng, Y. Shang, Z.-D. Yang and X. C. Zeng, J. Mater. Chem. C, 2014, 2, 10017–10030 RSC.
- J. C. Park, S. C. Yeo, D. H. Chun, J. T. Lim, J.-I. Yang, H.-T. Lee, S. Hong, H. M. Lee, C. S. Kim and H. Jung, J. Mater. Chem. A, 2014, 2, 14371–14379 CAS.
- X. Wang, P. Zhang, W. Wang, X. Lei, B. Zou and H. Yang, RSC Adv., 2015, 5, 27857–27861 RSC.
- R. Michalsky, Y.-J. Zhang, A. J. Medford and A. A. Peterson, J. Phys. Chem. C, 2014, 118, 13026–13034 CAS.
- P. Thüne, P. Moodley, F. Scheijen, H. Fredriksson, R. Lancee, J. Kropf, J. Miller and J. W. Niemantsverdriet, J. Phys. Chem. C, 2012, 116, 7367–7373 Search PubMed.
- J. Zhang, D. He, H. Su, X. Chen, M. Pan and S. Mu, J. Mater. Chem. A, 2014, 2, 1242–1246 CAS.
- X. Tian, T. Wang, Y. Yang, Y.-W. Li, J. Wang and H. Jiao, Phys. Chem. Chem. Phys., 2014, 16, 26997–27011 RSC.
- C. Yang, H. Zhao, Y. Hou and D. Ma, J. Am. Chem. Soc., 2012, 134, 15814–15821 CrossRef CAS PubMed.
- I. K. Herrmann, R. N. Grass, D. Mazunin and W. J. Stark, Chem. Mater., 2009, 21, 3275–3281 CrossRef CAS.
- G. Huang, J. Hu, H. Zhang, Z. Zhou, X. Chi and J. Gao, Nanoscale, 2014, 6, 726–730 RSC.
- V. Davydov, A. Rakhmanina, I. Kireev, I. Alieva, O. Zhironkina, O. Strelkova, V. Dianova, T. D. Samani, K. Mireles, L. H. Yahia, R. Uzbekov, V. Agafonov and V. Khabashesku, J. Mater. Chem. B, 2014, 2, 4250–4261 RSC.
- Y. Hu, J. O. Jensen, W. Zhang, L. N. Cleemann, W. Xing, N. J. Bjerrum and Q. Li, Angew. Chem., Int. Ed., 2014, 53, 3675–3679 CrossRef CAS PubMed.
- J. Blanchard and N. Abatzoglou, Catal. Today, 2014, 237, 150–156 CrossRef CAS PubMed.
- U. I. Kramm, I. Herrmann-Geppert, S. Fiechter, G. Zehl, I. Zizak, I. Dorbandt, D. Schmei and P. Bogdanoff, J. Mater. Chem. A, 2014, 2, 2663–2670 Search PubMed.
- X. Zhao, D. Xia, J. Yue and S. Liu, Electrochim. Acta, 2014, 116, 292–299 Search PubMed.
- R. Wang, H. Wang, H. Li, W. Wang, J. Key, L. Khotseng and S. Ji, Electrochim. Acta, 2014, 132, 251–257 Search PubMed.
- Z. Schnepp, S. C. Wimbush, M. Antonietti and C. Giordano, Chem. Mater., 2010, 22, 5340–5344 Search PubMed.
- V. Biju, Chem. Soc. Rev., 2014, 43, 744–764 Search PubMed.
- E. C. Vermisoglou, E. Devlin, T. Giannakopoulou, G. Romanos, N. Boukos, V. Psycharis, C. Lei, C. Lekakou, D. Petridis and C. Trapalis, J. Alloys Compd., 2014, 590, 102–109 Search PubMed.
- Y. Hu, J. O. Jensen, W. Zhang, Y. Huang, L. N. Cleemann, W. Xing, N. J. Bjerrum and Q. Li, ChemSusChem, 2014, 7, 2099–2103 Search PubMed.
- Y. Liao, K. Pan, L. Wang, Q. Pan, W. Zhou, X. Miao, B. Jiang, C. Tian, G. Tian, G. Wang and H. Fu, ACS Appl. Mater. Interfaces, 2013, 5, 3663–3670 Search PubMed.
- D. Amara and S. Margel, Colloid Polym. Sci., 2013, 291, 2121–2129 Search PubMed.
- M. Sugimoto, J. Am. Ceram. Soc., 1999, 82, 269–280 Search PubMed.
- H.-M. Fan, J.-B. Yi, Y. Yang, K.-W. Kho, H.-R. Tan, Z.-X. Shen, J. Ding, X.-W. Sun, M. C. Olivo and Y.-P. Feng, ACS Nano, 2009, 3, 2798–2808 Search PubMed.
- S. Phumying, S. Phokha and S. Maensiri, J. Supercond. Novel Magn., 2014, 27, 2573–2579 Search PubMed.
- K. S. Ranjith, P. Saravanan, S.-H. Chen, C.-L. Dong, C. L. Chen, S.-Y. Chen, K. Asokan and R. T. Rajendra Kumar, J. Phys. Chem. C, 2014, 118, 27039–27047 Search PubMed.
- Z. Q. Lv, W. T. Fu, S. H. Sun, X. H. Bai, Y. Gao, Z. H. Wang and P. Jiang, J. Magn. Magn. Mater., 2011, 323, 915–919 Search PubMed.
- C. X. Wang, Z. Q. Lv, W. T. Fu, Y. Li, S. H. Sun and B. Wang, Solid State Sci., 2011, 13, 1658–1663 Search PubMed.
- Y. Gao, Z. Lv, S. Sun, M. Qu, Z. Shi, R. Zhang and W. Fu, Mater. Lett., 2013, 100, 170–172 Search PubMed.
- M. Lei, H. Z. Zhao, H. Yang, B. Song and W. H. Tang, J. Eur. Ceram. Soc., 2008, 28, 1671–1677 Search PubMed.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 Search PubMed.
- H. Fjellvaag and P. Karen, Inorg. Chem., 1992, 31, 3260–3263 Search PubMed.
- Q. Su, J. Li, G. Du and B. Xu, J. Phys. Chem. C, 2012, 116, 23175–23179 Search PubMed.
- X. Wang, P. Zhang, J. Gao, X. Chen and H. Yang, Dyes Pigm., 2015, 112, 305–310 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra08618c |
| This journal is © The Royal Society of Chemistry 2015 |