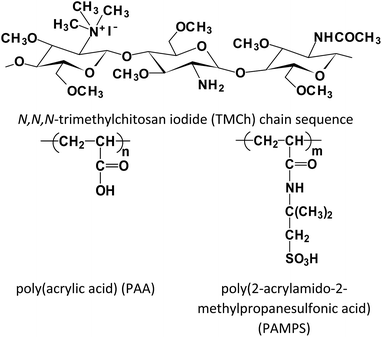
Novel antibacterial electrospun materials based on polyelectrolyte complexes of a quaternized chitosan derivative†
Kalin N.
Kalinov
a,
Milena G.
Ignatova
a,
Nevena E.
Manolova
*a,
Nadya D.
Markova
b,
Daniela B.
Karashanova
c and
Iliya B.
Rashkov
a
aLaboratory of Bioactive Polymers, Institute of Polymers, Bulgarian Academy of Sciences, Acad. G. Bonchev St, Bl. 103A, BG-1113 Sofia, Bulgaria. E-mail: manolova@polymer.bas.bg
bInstitute of Microbiology, Bulgarian Academy of Sciences, Acad. G. Bonchev Bl. 26, BG-1113 Sofia, Bulgaria
cInstitute of Optical Materials and Technologies, Bulgarian Academy of Sciences, Acad. G. Bonchev St, bl. 109, BG-1113 Sofia, Bulgaria
First published on 15th June 2015
Abstract
Novel nanofibrous materials composed of polyelectrolyte complexes (PECs) between N,N,N-trimethylchitosan iodide (TMCh) and poly(acrylic acid) (PAA) or poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS) were prepared. This was achieved by facile one-pot electrospinning of solutions of the oppositely charged polyelectrolyte partners. It was rendered possible by using a solvent system containing formic acid and/or by adding a strongly ionized low-molecular-weight salt (CaCl2). Use of formic acid enabled TMCh/PAA nanofibers containing in situ synthesized silver nanoparticles (AgNPs) to be electrospun. The AgNPs had an average diameter of 3.0 ± 0.8 nm and were uniformly distributed in the nanofibers as evidenced by the performed transmission electron microscopic (TEM) analyses. The prepared nanofibers preserved their morphology and did not dissolve in phosphate-buffered saline (PBS). Hybrid AgNPs-containing PEC nanofibrous materials showed good antibacterial activity against Gram-positive bacteria Staphylococcus aureus and Gram-negative bacteria Escherichia coli and possessed higher efficacy than that of the nanofibers of the same composition without AgNPs and TMCh.
1. Introduction
The quaternized derivative of chitosan – N,N,N-trimethylchitosan (TMCh) – like chitosan, is a biodegradable and biocompatible polymer exhibiting good antimicrobial properties. TMCh has some advantages over chitosan – it is soluble in neutral and alkaline media, it possesses ammonium groups in addition to amino groups, and exhibits higher efficacy against bacteria and fungi at pH ≥ 5.5.1–3 These properties render it particularly attractive for a number of biomedical applications. The utilization of the promising electrospinning technique represents an original approach for the preparation of TMCh-based polymer materials composed of continuous polymer fibers with diameters in the nanoscale range. One of the advantages of electrospinning is the facile fabrication of nanofibers loaded with low-molecular-weight biologically active substances such as drugs, or inorganic nanoparticles. We have shown that continuous TMCh-containing micro- and nanofibers are successfully prepared only when mixed solutions are electrospun – TMCh and a non-ionogenic water-soluble polymer – poly(vinyl alcohol) or poly(vinyl pyrrolidone) or TMCh and polyester – poly(L-lactide-co-D,L-lactide).4–6 Micro- and nanofibrous materials of this type possess good antibacterial and antimycotic activity.The one-pot preparation of micro- and nanofibrous materials based on polyelectrolyte complexes (PECs) which are insoluble in acidic, neutral and alkaline media is a promising strategy for the fabrication of novel electrospun materials with targeted properties which can find potential applications in various areas – in drug delivery systems, tissue engineering, as wound dressing materials. TMCh can form PECs with natural and synthetic polyacids, such as alginate, carrageenan, hyaluronic acid, poly(aspartic acid), methacrylic acid copolymer.7–12 Recently, we have reported on formation of PEC of TMCh with poly(acrylic acid) (PAA) or poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS).13 Depending on the pH of the medium, the nature of the polyacid, the molar mass of polyelectrolytes, as well as polyelectrolyte ratio, etc., TMCh-based micro- and nanosized PEC materials could be prepared.13,14 Previously, some of us have reported the preparation of nanofibers composed of chitosan-based PECs by one-pot electrospinning of solutions containing oppositely charged polyelectrolytes.15,16 In order to prevent the complex formation between the oppositely charged partners in PEC a solvent system with a suitable composition and pH value was used and/or a strongly ionized low-molecular-weight salt was added. Nanofibers from TMCh-based PECs have not been reported so far.
The electrospun nanofibrous materials containing silver nanoparticles (AgNPs) have evoked considerable interest because AgNPs exhibit an intrinsic antimicrobial activity against a wide spectrum of pathogenic microorganisms and good biocompatibility with mammalian tissues.17 These materials are potential candidates for wound dressing materials.18 In recent years the preparation of hybrid nanofibrous materials from AgNPs and chitosan or its derivatives has attracted significant attention.19–25 To the best of our knowledge, no data about the preparation of TMCh-based nanofibers with embedded AgNPs are available so far.
Performing one-pot electrospinning of oppositely charged polyelectrolytes is a challenge. The aim of the present contribution was to study the possibility to prepare fibrous materials composed of PECs between TMCh and PAA or PAMPS by solution electrospinning. Hybrid TMCh/PAA/AgNPs nanofibrous materials were also prepared applying one-pot approach. The antibacterial activity of the obtained nanofibrous materials against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) was evaluated.
2. Experimental
2.1 Materials
PAA (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w 250
w 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) (Aldrich), CH3I (Fluka), NaI (Fluka), AMPS (Fluka), formic acid (85 wt%, Fluka), anhydrous CaCl2 (Fluka), all salts used for the redox-initiator system (Fe(NH4)2(SO4)2, Na2SO3, (NH4)2S2O8) was of analytical grade of purity and were used without further purification. AgNO3 (ACS reagent, ≥99.0%) was purchased from Sigma-Aldrich and used as received. Prior to use, N-methyl-2-pyrrolidone (NMP) (99.0%, Fluka) was distilled under reduced pressure. Chitosan (Aldrich) with an average viscometric molar mass of 380
000 g mol−1) (Aldrich), CH3I (Fluka), NaI (Fluka), AMPS (Fluka), formic acid (85 wt%, Fluka), anhydrous CaCl2 (Fluka), all salts used for the redox-initiator system (Fe(NH4)2(SO4)2, Na2SO3, (NH4)2S2O8) was of analytical grade of purity and were used without further purification. AgNO3 (ACS reagent, ≥99.0%) was purchased from Sigma-Aldrich and used as received. Prior to use, N-methyl-2-pyrrolidone (NMP) (99.0%, Fluka) was distilled under reduced pressure. Chitosan (Aldrich) with an average viscometric molar mass of 380![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a deacetylation degree of 80% (as determined by IR spectroscopy) was used. N,N,N-trimethylchitosan iodide (TMCh) was prepared from chitosan using a known procedure.1 The quaternization degree of TMCh380000 was determined by 1H NMR and by potentiometric titration with aqueous silver nitrate, using a working silver electrode and a reference calomel electrode. The quaternization degree was calculated from the intensity ratio of the signal at 3.39 ppm for –R–N+(CH3)2I− (where R
000 g mol−1 and a deacetylation degree of 80% (as determined by IR spectroscopy) was used. N,N,N-trimethylchitosan iodide (TMCh) was prepared from chitosan using a known procedure.1 The quaternization degree of TMCh380000 was determined by 1H NMR and by potentiometric titration with aqueous silver nitrate, using a working silver electrode and a reference calomel electrode. The quaternization degree was calculated from the intensity ratio of the signal at 3.39 ppm for –R–N+(CH3)2I− (where R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 or CH3) to the signals at δ 3.64–4.54 ppm for H-2, H-3, H-4, H-5, H-6, H-6′ (6H). This value (56%) is in very good agreement with the quaternization degree determined potentiometrically (57%). The degree of methylation of the –OH functions was determined from the intensity ratios of the signal of CH3–O at 3.50 and 3.42 ppm for OH at C-3 and C-6 positions, respectively, and the H-2, H-3, H-4, H-5, H-6, H-6′ (6H) signals at δ 3.64–4.54. The degree of methylation of H-3 and H-6 was 97%. The acetylation degree was 20% and the non-substituted amino groups content was 24% as calculated from 1H NMR spectra. PAMPS was prepared by free radical polymerization as previously described.26 The
CH2 or CH3) to the signals at δ 3.64–4.54 ppm for H-2, H-3, H-4, H-5, H-6, H-6′ (6H). This value (56%) is in very good agreement with the quaternization degree determined potentiometrically (57%). The degree of methylation of the –OH functions was determined from the intensity ratios of the signal of CH3–O at 3.50 and 3.42 ppm for OH at C-3 and C-6 positions, respectively, and the H-2, H-3, H-4, H-5, H-6, H-6′ (6H) signals at δ 3.64–4.54. The degree of methylation of H-3 and H-6 was 97%. The acetylation degree was 20% and the non-substituted amino groups content was 24% as calculated from 1H NMR spectra. PAMPS was prepared by free radical polymerization as previously described.26 The ![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) v of PAMPS (2 × 106) was determined in 5 N NaCl at 25 °C by an Ubbelohde capillary type viscometer using known values of the Mark-Houwink constants.27
v of PAMPS (2 × 106) was determined in 5 N NaCl at 25 °C by an Ubbelohde capillary type viscometer using known values of the Mark-Houwink constants.27
S. aureus 749 and E. coli 3588 were purchased from the National Bank for Industrial Microorganisms and Cell Cultures, Sofia, Bulgaria.
2.2 Preparation of electrospun TMCh/PAA and TMCh/PAMPS nanofibrous mats
TMCh/PAA spinning solutions were prepared by mixing 9 wt% TMCh solution in 85 wt% HCOOH with 9 wt% solution of PAA in 85 wt% HCOOH/distilled water (1/2 v/v) at weight ratio of TMCh![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAA = 1
PAA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar ratio [TMCh]/[AA] = 1
1 (molar ratio [TMCh]/[AA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The total polymer concentration in the mixed solutions was 9 wt%. TMCh/PAMPS nanofibers at weight ratio of TMCh/PAMPS 1.5
3). The total polymer concentration in the mixed solutions was 9 wt%. TMCh/PAMPS nanofibers at weight ratio of TMCh/PAMPS 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (molar ratio [TMCh]/[AMPS] = 1
1 (molar ratio [TMCh]/[AMPS] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were prepared by electrospinning of their mixed solutions in 85 wt% HCOOH in the presence of 1.5 wt% CaCl2 at total polymer concentration of 7 wt%. The mixed solutions were homogeneous and transparent. They were placed into a plastic syringe (5 mL) equipped with 20G needle. The positive lead of a direct current high voltage power supply was connected to the needle via alligator clips. The electrospinning solutions were delivered at a controlled flow rate (0.2 mL h−1), at a constant value of the applied voltage (46 kV) and constant tip-to-collector distance (12 cm). The fibers were collected onto an aluminum foil fixed to a rotating grounded drum (of diameter 45 mm) at a rotating speed of 1000 rpm. The electrospun fibrous mats were placed under reduced pressure overnight at 45 °C to remove any solvent residues.
1) were prepared by electrospinning of their mixed solutions in 85 wt% HCOOH in the presence of 1.5 wt% CaCl2 at total polymer concentration of 7 wt%. The mixed solutions were homogeneous and transparent. They were placed into a plastic syringe (5 mL) equipped with 20G needle. The positive lead of a direct current high voltage power supply was connected to the needle via alligator clips. The electrospinning solutions were delivered at a controlled flow rate (0.2 mL h−1), at a constant value of the applied voltage (46 kV) and constant tip-to-collector distance (12 cm). The fibers were collected onto an aluminum foil fixed to a rotating grounded drum (of diameter 45 mm) at a rotating speed of 1000 rpm. The electrospun fibrous mats were placed under reduced pressure overnight at 45 °C to remove any solvent residues.
Prior to electrospinning, the dynamic viscosity of the spinning solutions was measured using a Bookfield DV-II+ Pro programmable viscometer for cone/plate option equipped with a sample thermostated cup and a cone spindle, at 25 ± 0.1 °C. The accuracy of the viscometer was verified using mineral oil viscosity standard fluids (BEL Part no. B200 (viscosity 200 cP, 25 °C) and B2000 (2000 cP)) (Brookfield Engineering Labs., Inc.). The electrical resistance of the spinning solutions was measured in an electrolytic cell equipped with rectangular sheet platinum electrodes as previously described.4
2.3 Preparation of hybrid nanofibrous TMCh/PAA/AgNPs mats
To 9 wt% TMCh (0.45 g) solution in 85 wt% HCOOH (5 g), 0.135 g AgNO3 was added. After stirring for 30 min, solution of PAA (0.45 g) in 85 wt% HCOOH/distilled water (1/2 v/v) (5 g) was added. The electrospinning set up, the nozzle tip/collector distance, the applied voltage and the feed rate for TMCh/PAA/AgNO3 were the same as for TMCh/PAA spinning solutions.2.4 Characterization of the mats
The morphology of the prepared electrospun materials was analyzed by scanning electron microscopy (SEM). For this purpose, the mats were vacuum-coated with gold and examined by a Jeol JSM-5510 scanning electron microscope. The fiber morphology was estimated in terms of the criteria for complex evaluation of electrospun mats reported elsewhere28 using the Image J software program29 by measuring at least 30 fibers from each SEM image. TEM observations were carried out by JEM 2100 (JEOL Co. Ltd.) operating at a voltage of 200 kV. The samples were prepared by electrospinning on a copper grid. The energy-dispersive X-ray (EDX) Genesis Microanalysis System was used to carry out the elemental analysis.The surface chemical composition of the complex electrospun mats was assessed by XPS. The XPS measurements were carried out in the ultrahigh-vacuum (UHV) chamber of an ESCALAB-MkII (VG Scientific) electron spectrometer using Mg Kα excitation with a total resolution of ca. 1 eV. Energy calibration was performed using the C1s line at 285 eV as a reference. The high-resolution spectra were dissected by means of special deconvolution software package.
Attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectra were recorded using an IRAffinity-1 spectrophotometer (Shimadzu Co., Kyoto, Japan) equipped with a MIRacle™ ATR (diamond crystal, depth of penetration of the IR beam into the sample – about 2 μm) accessory (PIKE Technologies, USA).
In order to demonstrate the presence of quaternary ammonium groups on the fiber surface the TMCh/PAA(PAMPS) mats were immersed in 0.2 wt% aqueous solution of fluorescein dye for 24 h. Then the samples were rinsed several times with distilled water, air dried, and analyzed by fluorescence microscopy (Leika DM 500B, Wetzlar, Germany).
To determine the stability of electrospun TMCh/PAA and TMCh/PAMPS complex mats in neutral media, the mats were immersed in a PBS (pH 7.4) for 24 h. The treated samples were repeatedly rinsed with distilled water, freeze-dried and the fiber morphology was examined by SEM microscopy. In order to determine the weight losses of TMCh/PAA and TMCh/PAMPS complex mats in neutral media, the mats were immersed in a PBS for 24 h.
The swelling degree (α) of electrospun TMCh/PAA and TMCh/PAMPS nanofibers after 24 h in PBS was determined gravimetrically and was calculated from eqn (1):
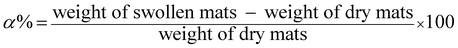 | (1) |
The stability of electrospun TMCh/PAA/AgNPs mats against PBS was determined by the same procedure as for TMCh/PAA and TMCh/PAMPS complex mats.
2.5 Antibacterial assessment
The antibacterial activity of the TMCh/PAA, TMCh/PAMPS and TMCh/PAA/AgNPs fibrous materials against S. aureus 749 and E. coli 3588 was evaluated by using the viable cell-counting method as described below. For comparison, the antibacterial activity of TMCh against both bacterial species was also assessed using aqueous solutions of the polymer. All electrospun mats were sterilized before the experiment by UV irradiation and after that they were exposed to bacterial suspension with concentration of 105 cells mL−1 prepared in test tubes with 5 mL of nutrient broth (Sigma-Aldrich). The test tubes were incubated at 37 °C for 24 h. Aliquots of 50 μL were taken from each test tube at different time intervals (30, 60, 120, 180 and 1440 min) and after ten-fold dilutions with sterile PBS, they were plated on Petri dishes with nutrient agar (Sigma-Aldrich). The plates were incubated at 37 °C for 24 h. The number of the surviving bacteria was determined by counting the colony forming units (CFU) in triplicate for each experiment.3. Results and discussion
3.1 Preparation and characterization of electrospun nanofibers based on TMCh/PAA and TMCh/PAMPS PECs
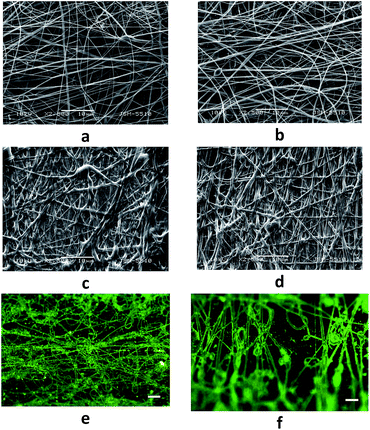
The one-pot solution electrospinning of oppositely charged polyelectrolytes is a challenge since it is necessary to overcome the possibility for formation of an insoluble PEC. In the present study, the optimal conditions for the preparation of electrospun nanofibers from the polycationic TMCh and PAA (weak polyacid) or PAMPS (strong polyacid) (Fig. 1) were searched for. Appropriate conditions were found for the preparation of a homogeneous common spinning solution containing the oppositely charged polyelectrolytes TMCh and PAA, i.e. without the occurrence of phase separation resulting from the formation of a water-insoluble PEC. In order to prevent the complex formation between the oppositely charged partners in PEC TMCh/PAA the 85 wt% HCOOH/H2O solvent system with a volume ratio of 1.3/0.7 was used. The use of this solvent provides the pH value of the solution to be ca. 1, i.e. the pH value is beyond the pH-range in which the oppositely charged polyelectrolytes are able to form a water-insoluble PEC. In our previous studies we have shown that for preparation of PEC nanofibers from chitosan and strong polyacid the addition of a low-molecular-weight salt is required.15 In the present study, when the strong polyacid PAMPS is used as a partner in the PEC TMCh/PAMPS for the preparation of a homogeneous common spinning solution the use of a 85 wt% HCOOH solvent system and the addition of a low molecular weight salt (1.5 wt% CaCl2) were found to be appropriate. It is known that low-molecular-weight salt contributes to the shielding of the charged functional groups of the polyelectrolytes and thus preventing the complex formation between them. For the TMCh/PAA system the selected [TMCh-units]/[AA-units] molar ratio was 1/3, because our previous studies had shown that at pH 4 a maximum amount of complex was formed at this ratio.13 For the TMCh/PAMPS system the [TMCh-units]/[AMPS-units] molar ratio of choice was 1/1, because we had previously demonstrated that at this ratio a maximum amount of TMCh/PAMPS complex was formed in the pH-range from 1 to 12.13 The preliminary experiments for optimizing the electrospinning conditions performed by varying the total polymer concentration from 6 to 10 wt%, the applied field strength (AFS) from 2.5 kV cm−1 to 4.2 kV cm−1 and the feeding rate from 0.1 to 0.6 mL h−1, indicated that cylindrical TMCh/PAA and TMCh/PAMPS fibers with a small number of spindle-like defects and a relatively narrow fiber diameter distribution were obtained at a total polymer concentration of 9 and 7 wt% for the TMCh/PAA and TMCh/PAMPS systems, respectively, a feeding rate of 0.2 mL h−1 and AFS 3.8 kV cm−1 (Fig. 2a and b). The TMCh/PAA fibers obtained under the above-stated electrospinning conditions had an average diameter of 300 ± 100 nm (Fig. 2b). A small number of spindle-like defects with an average size of 1430 × 4800 nm were formed. The average diameter of the obtained TMCh/PAMPS fibers was 210 ± 90 nm (Fig. 2a). A small number of spindle-like defects with an average size of 2400 × 5300 nm were formed. The observed slight decrease in the mean diameter of the TMCh/PAMPS fibers was due to the decrease in the solution viscosity from 2250 to 280 cP observed upon changing the polyanion in PEC from PAA to PAMPS and to the decrease in the total polymer concentration from 9 wt% (for TMCh/PAA) to 7 wt% (for TMCh/PAMPS). The electroconductivity of the solutions underwent an insignificant change from 4.6 mS cm−1 to 4.5 mS cm−1 for TMCh/PAA and TMCh/PAMPS, respectively.In order to study the stability of the fibers in aqueous medium TMCh/PAA and TMCh/PAMPS nanofibrous mats were immersed for 24 h in a PBS. The nanofibers swelled (Fig. 3c and d), but did not dissolve after 24 h immersion in the PBS. The average diameter of the nanofibers after 24 h stay, rinsing with distilled water and freeze-drying, increased from 300 ± 100 nm to 650 ± 170 nm for TMCh/PAA fibers and from 210 ± 90 nm to 760 ± 250 nm for TMCh/PAMPS fibers. The weight loss of the TMCh/PAMPS mat was equal to the amount of the incorporated CaCl2. The equilibrium swelling degree (αeq) determined in a PBS at 20 °C for TMCh/PAA and TMCh/PAMPS mats was 215 ± 7% and 305 ± 7%, respectively (Fig. 3).
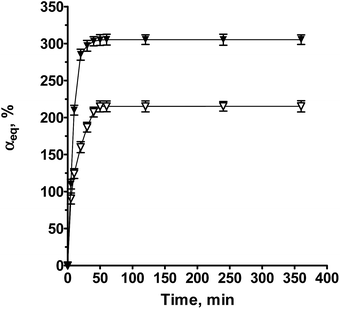 | ||
| Fig. 3 Equilibrium swelling degree (αeq), of TMCh380000/PAA (∇) and TMCh380000/PAMPS (▼) electrospun PEC nanofibers in a PBS versus time. | ||
The presence of quaternary ammonium groups on the surface of TMCh/PAA and TMCh/PAMPS mats was also demonstrated by treating the electrospun mats with an aqueous solution of fluorescein and subsequent observation of the mats by fluorescence microscopy (Fig. 2e and f). As seen in Fig. 2e and f, the nanofibrous mats showed fluorescence, which was due to the ionic interactions between the negatively charged carboxylate groups of the dye and the positively charged quaternary ammonium groups of TMCh.
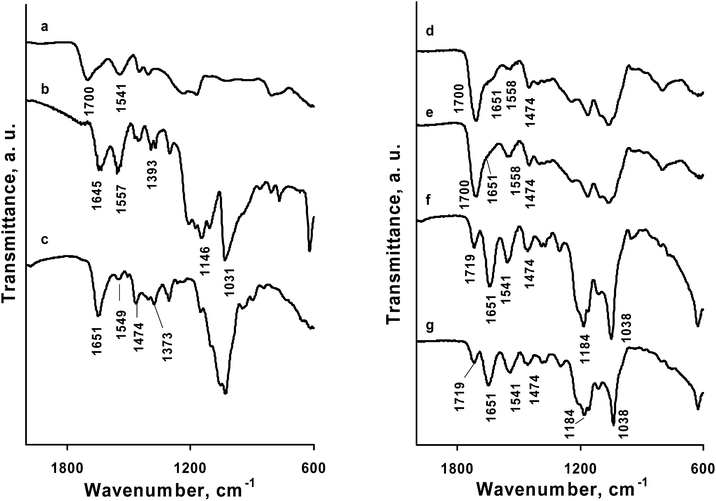
The nanofibers were characterized by ATR-FTIR spectroscopy before and after contact with water (Fig. 4). The ATR-FTIR spectra of TMCh/PAA and TMCh/PAMPS nanofibers before and after contact with water (Fig. 4d–g) showed the absorption bands characteristic of the two partners – TMCh (at 3440–3300 cm−1) (NH– and OH– stretching vibrations), 1651 cm−1 (amide I from the polysaccharide structure of TMCh) and 1474 cm−1 (stretching vibrations of the –CH3 and –CH2 groups located in the vicinity of the quaternary ammonium groups) and PAA or PAMPS. In the spectra of TMCh/PAA nanofibers (Fig. 4d and e) the band at 1700 cm−1, corresponding to stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations of the carboxyl group of PAA was detected, which overlapped the band at 1651 cm−1. New band appeared at 1558 cm−1, assigned to symmetrical stretching vibrations of carboxylate COO− groups of PAA. In the spectra of TMCh/PAMPS nanofibers (Fig. 4f and g) the bands assigned to the sulfonic acid groups, which were observed in the PAMPS spectrum at 1146 and 1031 cm−1, were shifted to higher wavenumber by 38 cm−1 and by 7 cm−1, respectively. The appearance of a new band at 1541 cm−1 characteristic of bending N–H vibrations of –NH3+ groups was observed as well as the appearance of a band at 1719 cm−1, which was attributed to complex formation of the amide groups from TMCh with Ca2+. The obtained results confirmed that the carboxyl groups of PAA are partially ionized to COO− groups, and the sulfo groups of PAMPS are ionized to SO3− groups, which form complexes with the protonated or quaternized amino groups of TMCh through electrostatic interactions. Moreover, after a stay in water the composition of the TMCh/PAA and TMCh/PAMPS nanofibrous mats remained unaltered.
O vibrations of the carboxyl group of PAA was detected, which overlapped the band at 1651 cm−1. New band appeared at 1558 cm−1, assigned to symmetrical stretching vibrations of carboxylate COO− groups of PAA. In the spectra of TMCh/PAMPS nanofibers (Fig. 4f and g) the bands assigned to the sulfonic acid groups, which were observed in the PAMPS spectrum at 1146 and 1031 cm−1, were shifted to higher wavenumber by 38 cm−1 and by 7 cm−1, respectively. The appearance of a new band at 1541 cm−1 characteristic of bending N–H vibrations of –NH3+ groups was observed as well as the appearance of a band at 1719 cm−1, which was attributed to complex formation of the amide groups from TMCh with Ca2+. The obtained results confirmed that the carboxyl groups of PAA are partially ionized to COO− groups, and the sulfo groups of PAMPS are ionized to SO3− groups, which form complexes with the protonated or quaternized amino groups of TMCh through electrostatic interactions. Moreover, after a stay in water the composition of the TMCh/PAA and TMCh/PAMPS nanofibrous mats remained unaltered.
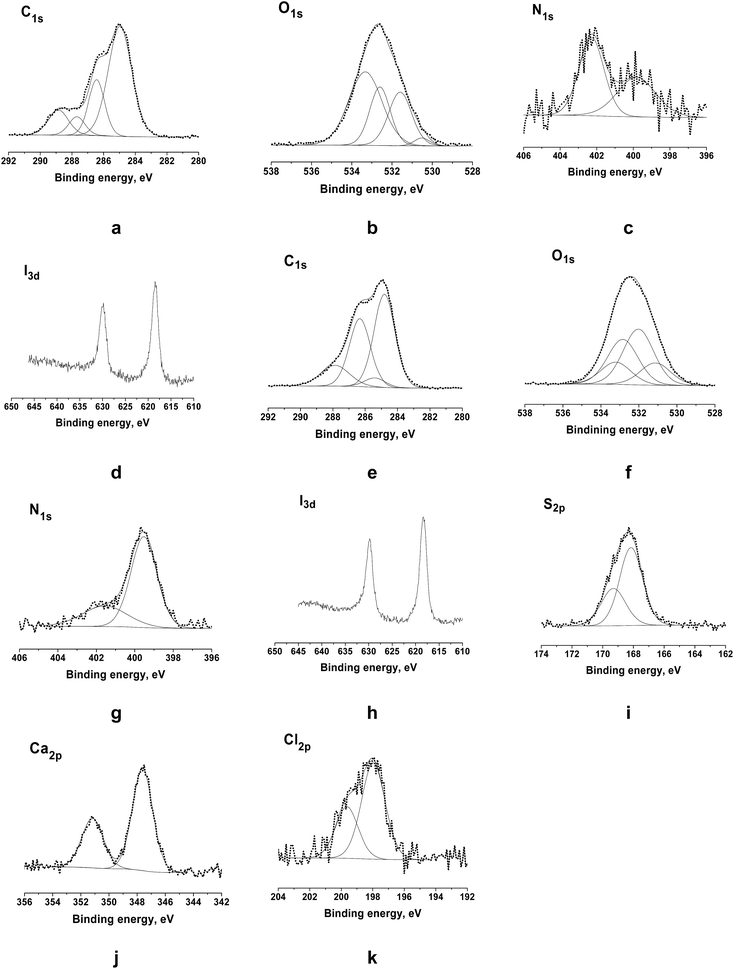
The expected structure of the nanofibers based on TMCh/PAA and TMCh/PAMPS PECs was also confirmed by XPS analysis. For the TMCh/PAA mat the constitutive atoms were detected by XPS (Fig. 5a–d): carbon (C1s) at 285 eV, oxygen (O1s) at 532.6 eV, nitrogen (N1s) at 399.8 and 402.3 eV and iodine (I3d) at 618.5 and 630 eV. Moreover, the C1s, O1s, N1s and I3d spectral regions were analyzed by peak reconstruction. The XPS spectrum of the C1s region of the TMCh/PAA mat is shown in Fig. 5a. Four C1s peaks were observed. The peak at 285 eV was assigned to –C–H or –C–C– from the TMCh and PAA partners and also to –C–NH2 from the TMCh partner, at 286.4 eV – to –C–O, –C–OH, –C–OCH3, –C–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the TMCh partner, at 287.7 eV – to –O–C–O– and –N–C
O of the TMCh partner, at 287.7 eV – to –O–C–O– and –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the TMCh partner and at 288.9 eV – to –O–C
O of the TMCh partner and at 288.9 eV – to –O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the PAA partner. In the detailed O1s spectrum shown in Fig. 5b four peaks were identified. The peak at 530.5 eV was assigned to –N–C
O of the PAA partner. In the detailed O1s spectrum shown in Fig. 5b four peaks were identified. The peak at 530.5 eV was assigned to –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the TMCh partner, the peak at 531.6 eV was ascribed to –C
O of the TMCh partner, the peak at 531.6 eV was ascribed to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the PAA partner, the peak at 532.6 eV was assigned to –C–OCH3 of the TMCh partner, and the peak at 533.3 eV was assigned to –O–C–O– of the TMCh partner and to O–C
O of the PAA partner, the peak at 532.6 eV was assigned to –C–OCH3 of the TMCh partner, and the peak at 533.3 eV was assigned to –O–C–O– of the TMCh partner and to O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the PAA partner. The expanded N1s spectrum showed two components – at 399.8 eV characteristic of –N–C
O of the PAA partner. The expanded N1s spectrum showed two components – at 399.8 eV characteristic of –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –NH2 and at 402.3 eV typical of the ammonium group (–N+(CH3)3)) from TMCh (Fig. 5c). The existence of peaks corresponding to N1s and I3d (at 618.5 eV (I3d5/2) and at 629.8 eV (I3d3/2) (Fig. 5d) confirmed the presence of TMCh in the surface layer of the TMCh/PAA mat.
O and –NH2 and at 402.3 eV typical of the ammonium group (–N+(CH3)3)) from TMCh (Fig. 5c). The existence of peaks corresponding to N1s and I3d (at 618.5 eV (I3d5/2) and at 629.8 eV (I3d3/2) (Fig. 5d) confirmed the presence of TMCh in the surface layer of the TMCh/PAA mat.
A confirmation for the successful incorporation of TMCh in the surface layer of TMCh/PAMPS PEC-based nanofibers was obtained from the performed XPS analyses. The appearance of N1s peaks was observed in the TMCh/PAMPS spectrum at 399.5 eV corresponding to –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –NH2 and at 401.6 eV characteristic of the –N+(CH3)3 group of TMCh (Fig. 5g). In addition, the spectrum showed the appearance of an I3d peak – at 618.4 eV (I3d5/2) and at 629.8 eV (I3d3/2), due to the presence of a TMCh component in the mat (Fig. 5h). Ca2p (at 351.2 eV (Ca2p1/2) and at 347.6 eV (Ca2p3/2) (Fig. 5j)) and Cl2p (at 199.7 eV (Cl2p1/2) and 198.0 eV (Cl2p3/2) (Fig. 5k)) peaks were detected, as well, attesting for the presence of CaCl2 in the surface layer of the TMCh/PAMPS mat. In the expanded C1s spectrum of the TMCh/PAMPS mat four peaks were identified (Fig. 5e). Taking into account the chemical structure of the mat the signal at 284.8 eV was assigned to –C–H or –C–C– from the TMCh and PAMPS partners as well as to –C–NH2 of the TMCh partner, and that at 286.3 eV – to –C–O, –C–OH, –C–OCH3, –C–N–C
O and –NH2 and at 401.6 eV characteristic of the –N+(CH3)3 group of TMCh (Fig. 5g). In addition, the spectrum showed the appearance of an I3d peak – at 618.4 eV (I3d5/2) and at 629.8 eV (I3d3/2), due to the presence of a TMCh component in the mat (Fig. 5h). Ca2p (at 351.2 eV (Ca2p1/2) and at 347.6 eV (Ca2p3/2) (Fig. 5j)) and Cl2p (at 199.7 eV (Cl2p1/2) and 198.0 eV (Cl2p3/2) (Fig. 5k)) peaks were detected, as well, attesting for the presence of CaCl2 in the surface layer of the TMCh/PAMPS mat. In the expanded C1s spectrum of the TMCh/PAMPS mat four peaks were identified (Fig. 5e). Taking into account the chemical structure of the mat the signal at 284.8 eV was assigned to –C–H or –C–C– from the TMCh and PAMPS partners as well as to –C–NH2 of the TMCh partner, and that at 286.3 eV – to –C–O, –C–OH, –C–OCH3, –C–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the TMCh partner and –C–NH–C
O of the TMCh partner and –C–NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the PAMPS partner. The peak at 287.9 eV was assigned to –O–C–O– and –N–C
O of the PAMPS partner. The peak at 287.9 eV was assigned to –O–C–O– and –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the TMCh partner and to –N–C
O of the TMCh partner and to –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the PAMPS partner. The signal for –C–SO3H of PAMPS appeared at 285.4 eV. The detailed O1s spectrum revealed four components – at 531.1 eV corresponding to –N–C
O of the PAMPS partner. The signal for –C–SO3H of PAMPS appeared at 285.4 eV. The detailed O1s spectrum revealed four components – at 531.1 eV corresponding to –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of TMCh and PAMPS, at 532.0 eV – assigned to –SO3H of the PAMPS partner, at 532.9 – to –C–OCH3 of the TMCh partner and at 533.2 – to –O–C–O– of the TMCh partner (Fig. 5f). A new S2p peak was also detected (at 169.3 eV (S2p1/2) and at 168.2 eV (S2p3/2)) corresponding to –SO3H of the PAMPS component in the fibers (Fig. 5i). These peaks are in agreement with the structure of the TMCh/PAMPS mat.
O of TMCh and PAMPS, at 532.0 eV – assigned to –SO3H of the PAMPS partner, at 532.9 – to –C–OCH3 of the TMCh partner and at 533.2 – to –O–C–O– of the TMCh partner (Fig. 5f). A new S2p peak was also detected (at 169.3 eV (S2p1/2) and at 168.2 eV (S2p3/2)) corresponding to –SO3H of the PAMPS component in the fibers (Fig. 5i). These peaks are in agreement with the structure of the TMCh/PAMPS mat.
3.2 Preparation and characterization of nanofibers composed of TMCh/PAA and AgNPs by one-pot electrospinning
In previous studies performed by some of us21,22 a novel approach for the preparation of water-insoluble hybrid nanofibrous materials based on chitosan or N-carboxyethylchitosan, polyoxyethylene and AgNPs by one-pot electrospinning was proposed. The ability of concentrated formic acid to dissolve chitosan or N-carboxyethylchitosan and polyoxyethylene, on the one hand, and to reduce Ag+ to Ag0, on the other hand, was used. In the present study we examined the possibility for preparing nanofibers from TMCh/PAA PEC loaded with AgNPs by one-pot electrospinning. In this case the same optimal conditions used for electrospinning of TMCh/PAA nanofibers were applied. The concentration of AgNO3 (0.08 mol L−1) was selected in such a manner as to ensure AgNPs content equal to 10 wt% of the total polymer weight. For stabilizing the AgNPs prepared in situ the following approach was applied – AgNO3 was added to the TMCh solution in 85 wt% HCOOH. 30 minutes after the addition of AgNO3 the TMCh solution turns brown which is indicative of the fact that a reduction of Ag+ ions to elemental silver has taken place. TMCh stabilized the obtained AgNPs. PAA was added subsequently to the spinning solution.The reduction of silver ions to elemental silver and AgNPs formation was followed by UV-Vis spectroscopy. The formation of AgNPs in the AgNO3 solution in formic acid was confirmed by the presence of an absorption band with a maximum at 415 nm characteristic of the surface plasmon resonance of AgNPs (Fig. S1a, ESI†). In the presence of TMCh the solution turned dark brown in 30 min and had a broader absorption band with a maximum at 430 nm (Fig. S1b, ESI†), which can be attributed to interactions between the polymer and the formed AgNPs, as well as to an increase in the AgNPs size.30,31
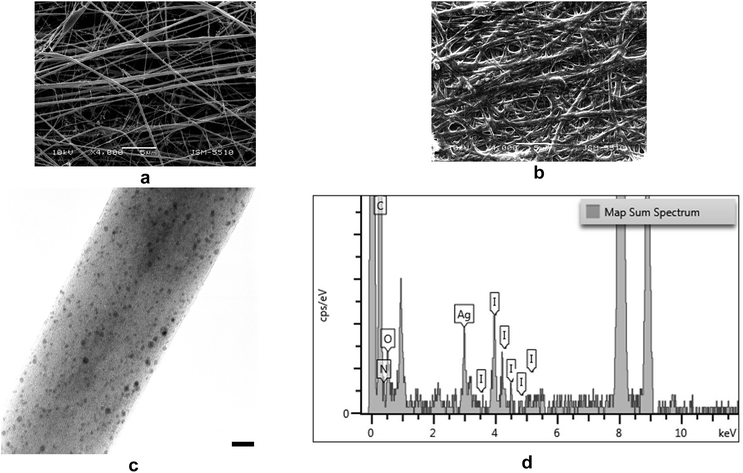
SEM and TEM micrographs of the prepared TMCh/PAA/AgNPs nanofibrous mats are shown in Fig. 6a and c. As seen from the SEM micrographs, the obtained fibers were cylindrical and contained spindle-like defects (Fig. 6a). The average fiber diameter (294 ± 100 nm) was close to that of the fibers prepared in the absence of AgNPs (300 ± 100 nm). A small number of spindle-like defects with an average size of 1200 × 4100 nm were formed. It was found that the nanofibrous TMCh/PAA/AgNPs mat preserved its integrity after a 24 h stay in PBS (Fig. 6b). Swelling, some coalescence of fibers and an increase in their average diameter – 610 ± 190 nm, was observed. AgNPs incorporated in the fibers were clearly visible in TEM micrographs due to the very low contrast of the polymer compared to that of AgNPs. As evident from the TEM micrographs, AgNPs were uniformly distributed along the fibers (Fig. 6c) and their average diameter was 3.0 ± 0.8 nm. The uniform distribution of AgNPs within the fibers attested for the good stabilization of the obtained AgNPs by TMCh and PAA. The EDX analyses of TMCh/PAA/AgNPs nanofibers showed characteristic peaks for C, O, N, I and Ag (Fig. 6d). The intensity of these peaks was essentially independent of the probed area, in consistence with the homogeneity of the fiber composition.
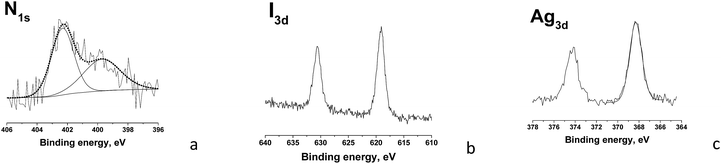
The composition of the surface layer of the hybrid TMCh/PAA/AgNPs nanofibers was analyzed by XPS. In the XPS spectrum of TMCh/PAA/AgNPs peaks for the expected C1s, O1s, N1s, I3d and Ag3d were observed. In Fig. 7 the deconvoluted N1s, I3d and Ag3d spectral regions of these nanofibers are shown. The presence of N1s peak consisted of two components – at 399.8 eV assigned to –N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and to –NH2 groups of TMCh and at 402.3 eV for the –N+(CH3)3 group of TMCh (Fig. 7a) and I3d peaks [I3d5/2 at 619.1 eV and I3d3/2 at 630.6 eV, Fig. 7b], proved the presence of TMCh in the surface layer of the nanofibers. In the N1s region the signal for nitrogen from nitrate ion (binding energy 406.6 ± 0.2 eV)32 was not registered, indicating the absence of unreacted AgNO3. The detailed Ag3d spectrum showed a couple of peaks separated by ca. 6 eV, corresponding to the spin–orbit splitting of the 3d level into 3d5/2 and 3d3/2. Ag3d5/2 signal is consisted of a peak at 368.3 eV and Ag3d3/2 signal is composed of a peak at 374.3 eV (Fig. 7c). The appearance of these peaks is indicative for the reduction of Ag+ to Ag0 and to the formation of AgNPs.33 The theoretically calculated and experimentally determined values of the oxygen, nitrogen, iodine and silver atomic percent contents are presented in Table 1. As seen from the Table, the experimentally determined values for the nitrogen and iodine contents were close to the feed ones. This was probably due to the presence of TMCh mainly on the surface of the fibers. The performed XPS analysis indicated that 14 wt% of AgNPs incorporated in the TMCh/PAA/AgNPs nanofibers were on their surface.
O and to –NH2 groups of TMCh and at 402.3 eV for the –N+(CH3)3 group of TMCh (Fig. 7a) and I3d peaks [I3d5/2 at 619.1 eV and I3d3/2 at 630.6 eV, Fig. 7b], proved the presence of TMCh in the surface layer of the nanofibers. In the N1s region the signal for nitrogen from nitrate ion (binding energy 406.6 ± 0.2 eV)32 was not registered, indicating the absence of unreacted AgNO3. The detailed Ag3d spectrum showed a couple of peaks separated by ca. 6 eV, corresponding to the spin–orbit splitting of the 3d level into 3d5/2 and 3d3/2. Ag3d5/2 signal is consisted of a peak at 368.3 eV and Ag3d3/2 signal is composed of a peak at 374.3 eV (Fig. 7c). The appearance of these peaks is indicative for the reduction of Ag+ to Ag0 and to the formation of AgNPs.33 The theoretically calculated and experimentally determined values of the oxygen, nitrogen, iodine and silver atomic percent contents are presented in Table 1. As seen from the Table, the experimentally determined values for the nitrogen and iodine contents were close to the feed ones. This was probably due to the presence of TMCh mainly on the surface of the fibers. The performed XPS analysis indicated that 14 wt% of AgNPs incorporated in the TMCh/PAA/AgNPs nanofibers were on their surface.
| Atom, % | Theoretically determined valuea | Experimentally determined valueb |
|---|---|---|
| a Theoretical values based on the weight of TMCh, PAA, and silver in the spinning solution used for the preparation of nanofibers. b Experimental values obtained from XPS analyses. The presented results are the average of three independent measurements. | ||
| O1s | 32.8 | 27.1 |
| N1s | 2.3 | 2.3 |
| I3d | 11.9 | 11.1 |
| Ag3d | 10 | 1.4 |
3.3 Assessment of the antibacterial activity of TMCh/PAA, TMCh/PAMPS and TMCh/PAA/AgNPs nanofibrous materials
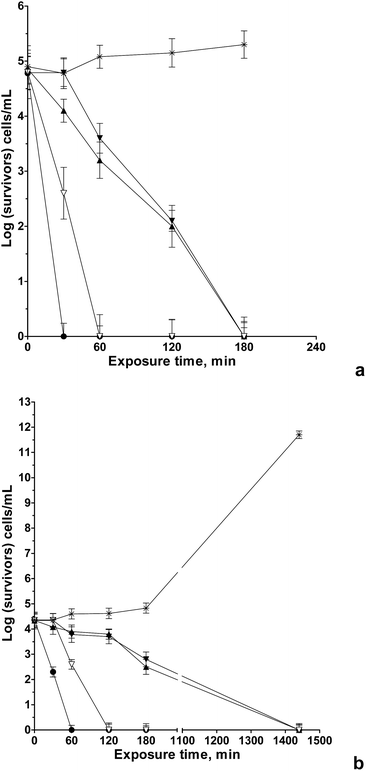
Antibacterial activity (bacteriostatic or bactericidal) of the nanofibrous TMCh/PAA, TMCh/PAMPS and TMCh/PAA/AgNPs materials against S. aureus and E. coli was tested in liquid medium after exposure for certain time intervals. The number of survived bacteria was subsequently assessed by plating and counting of CFU in solid medium. For comparison the activity of the TMCh solution was also assessed. It was found that the TMCh solution with a concentration of 3000 μg mL−1 displayed bactericidal activity against S. aureus. In this case, viable bacteria were absent after 60 min of incubation. As seen from Fig. 8a, S2b and c, ESI,† nanofibrous PEC TMCh/PAA (PAMPS) materials killed all bacteria within 180 min incubation, i.e. these materials exhibited bactericidal effect against S. aureus which is pronounced more slowly than that of TMCh. This could be accounted for the partial engagement of the positive charges of TMCh in complexation with PAA (PAMPS). These results are in accordance with previously reported results on the antibacterial activity of PEC.13,34 As seen in Fig. 8a, the TMCh/PAA/AgNPs materials had the most pronounced bactericidal activity – all the S. aureus cells were killed after coming into contact with nanofibrous materials containing 10 wt% AgNPs in 30 min.It was found that TMCh and all tested nanofibrous materials inhibited the growth of E. coli more slowly than S. aureus (Fig. 8b).
In the microbiological test against E. coli, the TMCh content was 7000 μg mL−1. In the case of PEC TMCh/PAA (PAMPS) mats all the E. coli were killed within 24 h of incubation (Fig. 8b and S3, ESI†). Incorporation of AgNPs in the TMCh/PAA fibrous materials led to a decrease of the bacterial titer to zero in 60 min of contact. The TMCh solution caused complete inhibition of the E. coli growth in 120 min. The observed considerable bactericidal activity of the hybrid nanofibrous TMCh/PAA/AgNPs materials may be attributed to combination of the high antibacterial activity of TMCh and that of AgNPs, manifesting itself in contact with the bacteria and the bactericidal activity of the released silver ions, respectively.
4. Conclusions
Novel water-insoluble nanofibrous materials from PEC TMCh/PAA and TMCh/PAMPS, as well as hybrid materials based on AgNPS-containing TMCh/PAA were prepared by one-pot electrospinning. Concentrated formic acid was used in order to: (i) dissolve TMCh, PAA, PAMPS and AgNO3, (ii) to prevent the interaction of the oppositely charged polyelectrolyte partners and (iii) to provide a mild reduction of silver ions. TMCh/PAA (PAMPS) and TMCh/PAA/AgNPs nanofibrous mats retained their integrity under conditions similar to those of human body fluids. This characteristic feature in combination with high antibacterial activity render the prepared nanofibrous materials suitable for a number of applications in the biomedical field, e.g., as wound dressing materials.Acknowledgements
The authors acknowledge Grant No 316086, FP7-REGPOT-2012-2013 and Dr. G. Lalev, School of Chemistry, Cardiff University, UK, for contribution to the EDX analyses. K.K. acknowledges the OP-HRD Grant BG051PO001-3.3.06-006 of the ESF.References
- C. H. Kim, J. W. Choi, H. J. Chun and K. S. Choi, Polym. Bull., 1997, 38, 387 CrossRef CAS.
- Z. Jia, D. Shen and W. Xu, Carbohydr. Res., 2001, 333, 1 CrossRef CAS PubMed.
- T. Xu, M. Xin, M. Li, H. Huang and S. Zhou, Carbohydr. Polym., 2010, 81, 931 CrossRef CAS.
- M. Ignatova, K. Starbova, N. Markova, N. Manolova and I. Rashkov, Carbohydr. Res., 2006, 341, 2098 CrossRef CAS PubMed.
- M. Ignatova, N. Manolova and I. Rashkov, Eur. Polym. J., 2007, 43, 1112 CrossRef CAS.
- M. Ignatova, N. Manolova, N. Markova and I. Rashkov, Macromol. Biosci., 2009, 9, 102 CrossRef CAS PubMed.
- K. Juntaprama, N. Praphairaksit, K. Siraleartmukul and N. Muangsin, Carbohydr. Polym., 2012, 90, 1469 CrossRef PubMed.
- R. J. Verheul, B. Slütter, S. M. Bal, J. A. Bouwstra, W. Jiskoot and W. E. Hennink, J. Controlled Release, 2011, 156, 46 CrossRef CAS PubMed.
- A. F. Martins, J. F. Piai, I. T. A. Schuquel, A. F. Rubira and E. C. Muniz, Colloid Polym. Sci., 2011, 289, 1133 CAS.
- B. Sayın, S. Somavarapu, X. W. Li, D. Sesardic, S. Senel and O. H. Alpar, Eur. J. Pharm. Sci., 2009, 38, 362 CrossRef PubMed.
- T. W. Wang, Q. Xu, Y. Wu, A. J. Zeng, M. Li and H. Gao, Carbohydr. Res., 2009, 344, 908 CrossRef CAS PubMed.
- V. Dehousse, N. Garbacki, A. Colige and B. Evrard, Biomaterials, 2010, 31, 1839 CrossRef CAS PubMed.
- K. Kalinov, M. Ignatova, N. Manolova, I. Rashkov, N. Markova and D. Momekova, Colloid Polym. Sci., 2014, 292, 2899 CAS.
- K. Kalinov, M. Ignatova, V. Maximova, I. Rashkov and N. Manolova, Eur. Polym. J., 2014, 50, 18 CrossRef CAS.
- H. Penchev, D. Paneva, N. Manolova and I. Rashkov, Macromol. Rapid Commun., 2008, 29, 677 CrossRef CAS.
- D. Paneva, N. Manolova, I. Rashkov, H. Penchev, M. Mihai and E. S. Dragan, Dig. J. Nanomater. Bios., 2010, 5, 811 Search PubMed.
- G. Franci, A. Falanga, S. Galdiero, L. Palomba, M. Rai, G. Morelli and M. Galdiero, Molecules, 2015, 20, 8856 CrossRef CAS PubMed.
- M. Ignatova, N. Manolova and I. Rashkov, Macromol. Biosci., 2013, 13, 860 CrossRef CAS PubMed.
- A. T. Hang, B. Tae and J. S. Park, Carbohydr. Polym., 2010, 82, 472 CrossRef CAS.
- T. T. T. Nguyen, B. Tae and J. S. Park, J. Mater. Sci., 2011, 46, 6528 CrossRef CAS.
- H. Penchev, D. Paneva, N. Manolova and I. Rashkov, Macromol. Biosci., 2009, 9, 884 CrossRef CAS PubMed.
- H. Penchev, D. Paneva, N. Manolova and I. Rashkov, Carbohydr. Res., 2010, 345, 2374 CrossRef CAS PubMed.
- H. T. Au, L. N. Pham, T. H. T. Vu and J. S. Park, Macromol. Res., 2012, 20, 51 CrossRef CAS.
- Y. Zhao, Y. Zhou, X. Wu, L. Wang, L. Xu and S. Wei, Appl. Surf. Sci., 2012, 258, 8867 CrossRef CAS.
- X. Wang, F. Cheng, J. Gao and L. Wang, J. Biomater. Appl., 2015, 29, 1086 CrossRef CAS PubMed.
- O. Stoilova, N. Koseva, N. Manolova and I. Rashkov, Polym. Bull., 1999, 43, 67 CrossRef CAS.
- L. Fisher, A. Sochor and J. Tan, Macromolecules, 1977, 10, 949 CrossRef CAS.
- M. Spasova, R. Mincheva, D. Paneva, N. Manolova and I. Rashkov, J. Bioact. Compat. Polym., 2006, 21, 465 CrossRef CAS.
- W. S. Rasband, ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2006, http://rsb.info.nih.gov/ij/ Search PubMed.
- M. H. El-Rafie, M. E. El-Naggar, M. A. Ramadan, M. M. G. Foudaa, S. S. Al-Deyab and A. Hebeish, Carbohydr. Polym., 2011, 86, 630 CrossRef CAS.
- Y.-K. Twu, Y.-W. Chen and C.-M. Shih, Powder Technol., 2008, 185, 251 CrossRef CAS.
- S. Jooyoung and J. Jyongsik, RSC Adv., 2013, 3, 22308 RSC.
- X. Liu, R. Jin, D. Chen, L. Chen, S. Xing, H. Xing, Y. Xing and Z. Su, J. Mater. Chem. A, 2015, 3, 4307 CAS.
- E. Yancheva, D. Paneva, V. Maximova, L. Mespouille, P. Dubois, N. Manolova and I. Rashkov, Biomacromolecules, 2007, 8, 976 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV-Vis spectra of AgNPs prepared in 85% HCOOH in the presence or absence of TMCh and digital photographs of antibacterial activity against S. aureus and E. coli of PEC nanofibrous materials with or without AgNPs. See DOI: 10.1039/c5ra08484a |
| This journal is © The Royal Society of Chemistry 2015 |