A pyrene-based optical probe capable of molecular computation using chemical input strings†‡
Megha Chhatwala,
Anup Kumara,
Rinkoo D. Gupta*b and
Satish K. Awasthi*a
aChemical Biology Laboratory, Department of Chemistry, University of Delhi, Delhi-110 007, India. E-mail: skawasthi@chemistry.du.ac.in
bFaculty of Life Sciences and Biotechnology, South Asian University, New Delhi-110 021, India
First published on 2nd June 2015
Abstract
A pyrene-based optical probe for the real-time and regenerative detection of Cu2+ and Fe3+ at parts-per-million (ppm) levels is demonstrated. Moreover, the quantifiable changes in the fluorescence signal induced by chemical inputs viz. Cu2+, Fe3+, H+ and CN− have been exploited to assemble sequential and “four-input” combinatorial molecular logic circuits. A unique “two-way” security lock has also been devised for enhanced information protection at the molecular level.
Multi-stimuli responsive1 “smart molecules” illustrate huge promises in the field of molecular computing. The perturbations caused in their immediate environment by the external stimuli (chemical, electrical and/or optical inputs) get amplified to the macroscopic level in terms of measurable signals (mechanical, optical and/or electrochemical outputs), which can be ingeniously harnessed for Boolean algebraic computations allowing information processing and storage at the molecular level.2 Chemosensors endowed with a pyrene moiety as the signalling unit generate either emission enhancement or quenching as the output in response to metal ion stimuli.3 In this context, we introduce a simple pyrene-based probe 1, for the detection of Cu2+, Fe3+ and H+ ions via discernible colour changes and discriminating emission “turn-off” behaviour. The 1-Cu2+/Fe3+ ensembles could further recognize highly toxic cyanide ion with simultaneous regeneration of probe 1. Furthermore, these ions (Cu2+, Fe3+, H+ and CN−) have been utilized as the input strings to construct molecular-level logic circuits while accessing quantum yield responses as outputs. To the best of our knowledge, there has been no report on any such pyrene-based chemosensor for recognition of all the four ions viz. Cu2+, Fe3+, CN− and H+ on a single platform, till date.4
Probe 1 (Scheme 1) has been synthesized in good yield (∼70%) by adopting a simple synthetic method5 and well-characterized using a full-battery of physico-chemical techniques (Fig. S1–S4‡). The availability of the carbonyl oxygen and pyridine nitrogen atoms makes our probe an enticing receptor for Cu2+, Fe3+ and H+ ions. The probe displays metal/H+-ion induced differential optical responses (UV-vis and emission) along with contrasting colour changes.
The UV-vis spectrum of 1 (10−5 M, CHCl3) displays an intense peak at λ = 270 nm (ε = 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and a broad doublet at λ = 392 nm (ε = 23
000 M−1 cm−1) and a broad doublet at λ = 392 nm (ε = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and 430 nm (ε = 26
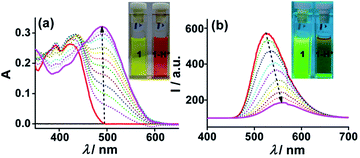
000 M−1 cm−1) and 430 nm (ε = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1). However, the spectrum shows immediate perturbations in the presence of Cu2+ and Fe3+ ions at ppm level concentration associated with visible colour changes from yellow to purple and blue, respectively (Fig. S5 and S6‡). Upon gradual addition of Cu2+ (1–20 ppm, CH3CN) to 1 (10−5 M, CHCl3), a moderate hypochromic shift at λ = 430 nm (“turn-off”, ΔA = 0.103) coupled with appearance of a new peak at λ = 567 nm (“turn-on”, ΔA = 0.352) was observed. Also, two isosbestic points at λ = 370 and 450 nm indicate the formation of 1-Cu2+ ensemble (Fig. 1a). Strikingly, addition of only a small concentration of Fe3+ (1–5 ppm, CH3CN) induced a significant hypochromic shift at λ = 430 nm (“turn-off”, ΔA = 0.188) together with an emergence of a broad band with dual peaks at λ = 540 (“turn-on”, ΔA = 0.125) and 611 nm (“turn-on”, ΔA = 0.128). An isosbestic point at λ = 466 nm accounts for the binding of Fe3+ with 1 (Fig. 1b). The spectral responses saturated at 20 ppm and 5 ppm for Cu2+ and Fe3+, respectively and no further remarkable changes were detected on increasing the concentration of analytes by two-fold.
000 M−1 cm−1). However, the spectrum shows immediate perturbations in the presence of Cu2+ and Fe3+ ions at ppm level concentration associated with visible colour changes from yellow to purple and blue, respectively (Fig. S5 and S6‡). Upon gradual addition of Cu2+ (1–20 ppm, CH3CN) to 1 (10−5 M, CHCl3), a moderate hypochromic shift at λ = 430 nm (“turn-off”, ΔA = 0.103) coupled with appearance of a new peak at λ = 567 nm (“turn-on”, ΔA = 0.352) was observed. Also, two isosbestic points at λ = 370 and 450 nm indicate the formation of 1-Cu2+ ensemble (Fig. 1a). Strikingly, addition of only a small concentration of Fe3+ (1–5 ppm, CH3CN) induced a significant hypochromic shift at λ = 430 nm (“turn-off”, ΔA = 0.188) together with an emergence of a broad band with dual peaks at λ = 540 (“turn-on”, ΔA = 0.125) and 611 nm (“turn-on”, ΔA = 0.128). An isosbestic point at λ = 466 nm accounts for the binding of Fe3+ with 1 (Fig. 1b). The spectral responses saturated at 20 ppm and 5 ppm for Cu2+ and Fe3+, respectively and no further remarkable changes were detected on increasing the concentration of analytes by two-fold.
Furthermore, probe 1 is an intense luminophore (ϕf = 0.62, using anthracene as standard) and exhibits an emission peak at λ = 520 nm (λex = 270 nm). Plausibly, the peak can be assigned to intermolecular excimer emission due to π–π stacking of pyrene units.3a Interestingly, upon addition of 20 ppm of Cu2+ (∼8.3 equiv.), 1 showed “turn-off” emission response with I0/I = ∼7 (ϕf(1-Cu2+) = 0.09) and a miniscule concentration of 5 ppm (∼3.1 equiv.) of Fe3+ also led to diminishing of the emission peak with I0/I = ∼13 (ϕf(1-Fe3+) = 0.05) (Fig. 2). The quenching could also be visualized under UV lamp (Fig. S7‡). The fluorescence quenching is attributed to the non-radiative processes caused by paramagnetic effect of unpaired d-electrons of Cu2+ (d9) and Fe3+ (d5).6 The Stern–Volmer plots were non-linear with an upward curvature indicating both static (collisional) as well as dynamic (formation of non-fluorescent ground state complex) quenching mechanisms operating together (Fig. S8‡).7
The detection limits of 1 for Cu2+ and Fe3+ came out to be 4.4 × 10−6 M (∼1 ppm) and 2.3 × 10−6 M (∼0.4 ppm), respectively.8a The stoichiometric ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for both 1-Cu2+ and 1-Fe3+ couples was evaluated from Job's method of continuous variation of mole-fraction (Fig. S9‡). The binding ratio has also been confirmed by ESI-MS data. A clear peak at m/z = 729.79 may be attributed to the fragment [2.probe1 + Cu] (calc. m/z = 729.16) (Fig. S10‡) and a peak at m/z = 304.86 most likely corresponds to the fragment [2.probe1 + Fe(CH3CN)2·6H2O]3+ (calc. m/z = 304.09) (Fig. S11‡). The binding constants computed from Benesi–Hildebrand plots assuming 2
1 for both 1-Cu2+ and 1-Fe3+ couples was evaluated from Job's method of continuous variation of mole-fraction (Fig. S9‡). The binding ratio has also been confirmed by ESI-MS data. A clear peak at m/z = 729.79 may be attributed to the fragment [2.probe1 + Cu] (calc. m/z = 729.16) (Fig. S10‡) and a peak at m/z = 304.86 most likely corresponds to the fragment [2.probe1 + Fe(CH3CN)2·6H2O]3+ (calc. m/z = 304.09) (Fig. S11‡). The binding constants computed from Benesi–Hildebrand plots assuming 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molecular arrangement were 1.72 × 102 M−1/2 and 1.94 × 103 M−1/2 for Cu2+ and Fe3+, respectively (Fig. S12‡).8b
1 molecular arrangement were 1.72 × 102 M−1/2 and 1.94 × 103 M−1/2 for Cu2+ and Fe3+, respectively (Fig. S12‡).8b
An excellent probe needs to demonstrate highly selective identification in the competitive environment of analytes. The chemosensor 1 exclusively detects Cu2+ and Fe3+ over a wide range of other metal ions. No other metal ion could produce any remarkable change in the emission spectra of 1 (red bars). Furthermore, quenching efficiency of the probe for Cu2+ (blue bars) and Fe3+ (green bars) ions was not deterred even by the presence of other test stimuli in a matrix arrangement (Fig. 3). Apparently due to its higher binding constant, Fe3+ most likely replaces Cu2+ from 1-Cu2+ couple further decreasing the emission intensity (blue bars, entry 17, Fig. 3a).
Recyclability of a chemosensor is crucial for cost-effective real-sample measurements. Addition of 5 ppm of CN− (in ethanol) in the respective solutions of 1-Cu2+ and 1-Fe3+ complexes, not only reverted back the emission intensity of probe 1 but also brought back its initial yellow colour (Fig. 4a). This possibly implies sequestering of Cu2+ and Fe3+ by CN− to potentially form [Cu(CN)x]n− and [Fe(CN)x]n−, releasing probe 1 in the solution.9 None of the other investigated anions could remarkably raise the quenched fluorescence signal (Fig. 4b). Both 1-Cu2+ and 1-Fe3+ systems showed good on–off ratio for at least three cycles with only a minimal signal loss of ∼9% and ∼19%, respectively (Fig. S13‡). It is noteworthy to mention here, that probe 1 depicted an added advantage of detection of a potentially toxic stimulus (CN−).
Another feather in our probe's cap is its distinct optical response towards the presence of H+ ions. Upon gradual addition of H+ ions (0.3 mM to 2 mM, CH3CN) to the solution of 1 (10−5 M, CHCl3), a new absorption band arises at λ = 488 nm (“turn-on”, ΔA = 0.313) along with fading of the original bands. This could be ascribed to the protonation of pyridine nitrogen atom. The response was visually detectable with a colour change from yellow to orange. On the other hand, the emission peak plummets (I0/I = ∼3.5, ϕf(1-H+) = 0.18) coupled with the shifting of λmax to 550 nm (bathochromic shift of ∼30 nm) as H+ ions (0.04 mM to 2 mM, CH3CN) are introduced into the system (Fig. 5).
As explained, probe 1 acts as an “ON–OFF–ON” fluorescent switch controlled by Cu2+/Fe3+ and CN− ions (vide supra). The set-reset of probe 1 by these stimuli can process information in the form of two-input sequential and combinatorial logic circuits.10 The variations in the quantum yield at λ = 520 nm have been captured as outputs and the threshold has been fixed at ϕf = 0.3. The sequential logic circuit is designed in such a way that Cu2+ ion behaves as IN1 whereas CN− ion functions as IN2 (Fig. 6a). When IN1 is high, the quantum yield falls below the threshold level to give OUT = 0 and the stored information is “erased” from the system. However, subsequent addition of IN2 again “writes” the information in the system as it regains its initial quantum yield with OUT = 1. The feedback loop connects the output back to IN1 and ensures memory function of the circuit. When the sequence order of the inputs is reversed and CN− ion (IN1) is added before Cu2+ ion (IN2), a combinatorial logic circuit mimicking the functions of NOT, AND and OR gates, is obtained (Fig. 6b). In this case, already present CN− ions (IN1 = 1) in the system could not sequester the metal ion (IN2 = 1) and thus, the information is “read” in terms of low output (OUT = 0). These logic systems could also be concatenated for Fe3+ ion.
Evidently, the above used input events are order dependent and generate TRUE output only when addition of Cu2+/Fe3+ precedes that of CN−. Hence, the system more or less behaves like a priority AND gate. This modulation in the input sequence could also be realised to devise a miniaturized “two-way” security lock11 for information cascade at molecular level. The inputs Cu2+, Fe3+ and CN− ions are coded as “U”, “S” and “B”, respectively. Out of six possible combinations (USB, BSU, SBU, UBS, SUB and BUS), the input sequence “USB” unlocks the emission signal at λ = 520 nm.
Markedly, the positions of inputs “U” and “S” could be dexterously interchanged with each other to generate another input sequence “SUB” which could also turn-on the emission signal. Thus, this “joint molecular account” is held by two authorized users who equally share the right to activate the emission channel by entering their respective coded sequence of keys (either “USB” or “SUB”). Any other key string, if pressed would fail to open the lock and produce an alarm (FALSE) signal. (Fig. 7).
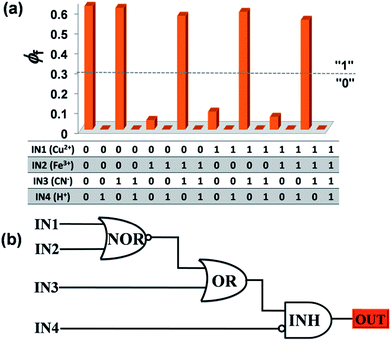
Further, sixteen different combinations of four chemical inputs viz. Cu2+ (IN1), Fe3+ (IN2), CN− (IN3) and H+ (IN4) were used to address the emission quantum yield outputs at λ = 520 nm (OUT). The threshold has been set at ϕf = 0.30. OUT is generated by a four-input combinatorial circuit comprising of NOR, OR and INH gates wired together. (Fig. 8). Therefore, the fluorogenic sensing traits of probe 1 can be usefully extended to configure molecular level logic circuits to store the optical information encoded by appropriate chemical input signals.
Conclusions
In conclusion, we have presented a simple pyrene-appended chemosensor for differential monitoring of Cu2+, Fe3+ and H+ ions via distinct colour changes and emission quenching. The 1-Cu2+/Fe3+ couples formed in situ could further detect toxic CN− ions while reverting the probe 1 back into the solution. Most importantly, the probe adds further impetus to the field of molecular devices while performing Boolean algebraic operations to integrate two-input sequential as well as combinatorial logic circuits by controlling the sequence of input addition. Additionally, a complex four-input combinatorial logic circuit could also be achieved. Thus, probe 1 offers highly selective, sensitive and reversible detection of Cu2+ and Fe3+ and also opens new avenues to explore the potential of chemosensors as memory elements.Acknowledgements
RDG thanks DST (SERB/F/1424/2013-14) and South Asian University, New Delhi, India for financial assistance. MC thanks CSIR, India for award of Senior Research Fellowship and University of Delhi for technical support.Notes and references
- (a) K. Chen, Q. Shu and M. Schmittel, Chem. Soc. Rev., 2015, 44, 136 RSC; (b) M. Chhatwal, A. Kumar, V. Singh, R. D. Gupta and S. K. Awasthi, Coord. Chem. Rev., 2015, 292, 30 CrossRef CAS PubMed; (c) S. Banthia and A. Samanta, Eur. J. Org. Chem., 2005, 4967 CrossRef CAS PubMed.
- (a) A. P. de Silva and N. D. McClenaghan, Chem.–Eur. J., 2004, 10, 574 CrossRef PubMed; (b) J. Andréasson and U. Pischel, Chem. Soc. Rev., 2015, 44, 1053 RSC; (c) A. Credi, Angew. Chem., Int. Ed., 2007, 46, 5472 CrossRef CAS PubMed; (d) J. Andréasson and U. Pischel, Chem. Soc. Rev., 2015, 44, 1053 RSC.
- (a) C. Kar, M. D. Adhikari, A. Ramesh and G. Das, RSC Adv., 2012, 2, 9201 RSC; (b) L. Ding, S. Wang, Y. Liu, J. Cao and Y. Fang, J. Mater. Chem., 2013, 1, 8866 RSC; (c) T. Raj, P. Saluja and N. Singh, Sens. Actuators, B, 2015, 206, 98 CrossRef CAS PubMed; (d) M. Kumar, A. Dhir and V. Bhalla, Eur. J. Org. Chem., 2009, 4534 CrossRef CAS PubMed; (e) M. Kumar, R. Kumar and V. Bhalla, Tetrahedron Lett., 2010, 51, 5559 CrossRef CAS PubMed.
- (a) E. Manandhar and K. J. Wallace, Inorg. Chim. Acta, 2012, 381, 15 CrossRef CAS PubMed; (b) Y. R. Bhorge, H. Tsaia, K. Huang, A. J. Pape, S. N. Janaki and Y. Yen, Spectrochim. Acta, Part A, 2014, 130, 7 CrossRef CAS PubMed; (c) N. Sharma, S. I. Reja, V. Bhalla and M. Kumar, Dalton Trans., 2014, 15929 RSC; (d) M. Wang, J. Xu, X. Liu and H. Wang, New J. Chem., 2013, 37, 3869 RSC.
- W. Leslie, R. A. Poole, P. R. Murray, L. J. Yellowlees, A. Beeby and J. A. G. Williams, Polyhedron, 2004, 23, 2769 CrossRef CAS PubMed.
- (a) A. W. Varnes, R. B. Dodson and E. L. Wehry, J. Am. Chem. Soc., 1972, 94, 946 CrossRef CAS; (b) S. K. Sahoo, D. Sharma, R. K. Bera, G. Crisponi and J. F. Callan, Chem. Soc. Rev., 2012, 41, 7195 RSC.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
- (a) A. Kumar, M. Chhatwal, R. D. Gupta and S. K. Awasthi, RSC Adv., 2015, 5, 5217 RSC; (b) S. Sarkar, S. Roy, A. Sikdar, R. N. Saha and S. S. Panja, Analyst, 2013, 138, 7119 RSC.
- P. Kaur, H. Kaur and K. Singh, RSC Adv., 2013, 3, 64 RSC.
- (a) G. de Ruiter, E. Tartakovsky, N. Oded and M. E. van der Boom, Angew. Chem., Int. Ed., 2010, 49, 169 CrossRef CAS PubMed; (b) S. Karmakar, D. Maity, S. Mardanya and S. Baitalik, J. Phys. Chem. A, 2014, 118, 9397 CrossRef CAS PubMed; (c) L. Wang, B. Li, L. Zhang and Y. Luo, Dalton Trans., 2013, 459 RSC; (d) M. A. Kaloo and J. Sankar, New J. Chem., 2014, 38, 923 RSC; (e) V. Bhalla, Roopa and M. Kumar, Dalton Trans., 2013, 13390 RSC; (f) M. Kumar, S. I. Reja and V. Bhalla, Org. Lett., 2012, 14, 6084 CrossRef CAS PubMed.
- (a) D. Margulies, C. E. Felder, G. Melman and A. Shanzer, J. Am. Chem. Soc., 2007, 129, 347 CrossRef CAS PubMed; (b) M. Suresh, A. Ghosh and A. Das, Chem. Commun., 2008, 3906 RSC; (c) M. Kumar, R. Kumar and V. Bhalla, Chem. Commun., 2009, 7384 RSC; (d) M. Kumar, A. Dhir and V. Bhalla, Org. Lett., 2009, 11, 2567 CrossRef CAS PubMed; (e) Y. Wang, Y. Huang, B. Li, L. Zhang, H. Song, H. Jiang and J. Gao, RSC Adv., 2011, 1, 1294 RSC; (f) V. Bhalla, V. Vij, M. Kumar, P. R. Sharma and T. Kaur, Org. Lett., 2012, 14, 1012 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Late Dr Tarkeshwar Gupta. |
| ‡ Electronic supplementary information (ESI) available: Experimental details, characterization and sensing methodology. See DOI: 10.1039/c5ra08465b |
| This journal is © The Royal Society of Chemistry 2015 |