Electrolytic recovery of bismuth and copper as a powder from acidic sulfate effluents using an emew® cell
Abstract
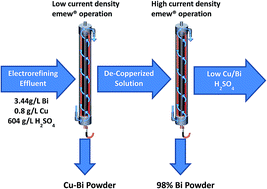
Effective removal of bismuth is a primary concern during copper electrorefining. A novel electrowinning process using an emew® cell was developed to recover bismuth and copper from a copper electrorefining waste stream. Significant removal and co-deposition of copper and bismuth were achieved from a highly acidic sulfate industrial effluent using a current density of 350 A m−2, but the current efficiency was low (27%). Operating at a lower current density (75 A m−2) facilitated the preferred removal of Cu, while increasing current efficiency to 67.4% due to the decrease of aside-reaction. Consequently, a two-stage process was employed to remove most of the copper at low current and then extract bismuth at high current. 93.4% of the bismuth and 97.8% of the copper were recovered with a satisfactory current efficiency, and a high purity (∼98%) Bi powder was obtained in the second step. This novel emew® cell approach may serve as a promising alternative for recovering copper and bismuth, and the proposed two step strategy may offer insight for the selective recovery of metals in a multi-component system.

 Please wait while we load your content...
Please wait while we load your content...