C-doped boron nitride fullerene as a novel catalyst for acetylene hydrochlorination: a DFT study†
Fei Zhao,
Yang Wang,
Mingyuan Zhu and
Lihua Kang*
College of Chemistry and Chemical Engineering/Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, Shihezi University, Shihezi, Xinjiang 832000, PR China. E-mail: kanglihua@shzu.edu.cn; Fax: +86-0993-2057270; Tel: +86-0993-2057213
First published on 19th June 2015
Abstract
Density functional theory calculations were used to investigate the mechanism of acetylene hydrochlorination separately catalyzed by un-doped B12N12 and carbon-doped BN fullerene (B12−nN11+nC (n = 0, 1)). We have discovered that carbon-doped BN clusters displayed extraordinary catalyst performance for acetylene hydrochlorination compared with un-doped B12N12 clusters. C2H2 was adsorbed onto B12−nN11+nC (n = 0, 1) clusters prior to HCl and then formed three adsorption states. The first two states were in a trans configuration, in which the two H atoms of C2H2 were on opposite sides of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond; the third state was a cis configuration, in which the two H atoms were on the same side of the C
C bond; the third state was a cis configuration, in which the two H atoms were on the same side of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. Afterwards, we illustrated three possible pathways with corresponding transition states. In particular, the minimum energy pathway R1 based on the B11N12C catalyst had an energy barrier as low as 36.08 kcal mol−1, with only one transition state.
C bond. Afterwards, we illustrated three possible pathways with corresponding transition states. In particular, the minimum energy pathway R1 based on the B11N12C catalyst had an energy barrier as low as 36.08 kcal mol−1, with only one transition state.
1. Introduction
Not long after the discovery of C60 by Kroto et al. in 1985,1 research on fullerene and related materials boomed. With the first case of successful preparation of doped fullerene,2 investigators have extensively researched the preparation, structure, and application of various doped fullerenes.3–11 Among these doped fullerenes, the B12N12 cage is considered to be the smallest stable cage.6,12,13 B12N12 cages were successfully synthesized by Oku et al. in 2004 and were analyzed by laser desorption time-of-flight mass spectrometry.6,14 Their research revealed that B12N12 clusters consisting of four- and six-membered BN rings satisfy the isolated tetragonal rule. Although scientists have widely explored the usage of these clusters as catalysts, many studies have concentrated on their application as hydrogen-storage materials8,9,15,16 and have neglected their potential roles in other reactions. In the present work, doped BN fullerene was used as a catalyst for acetylene hydrochlorination for the first time.Mercuric chloride is the most commonly used catalyst for the industrial production of vinyl chloride monomer. However, mercuric chloride catalyst easily sublimes at high temperatures17,18 and seriously harms human health and the environment. Therefore, possible alternatives that are efficient and environment friendly are necessary. In 1985,19 Hutchings et al. predicted that gold-based catalysts could be greener substitutes for mercuric chloride. They performed an experiment20 to confirm that Au3+ possesses the highest catalytic activity among the selected metal complexes containing Bi3+,21 Pd2+,22 Pt2+,23 and Pt4+.24 However, the supported gold catalysts were more easily deactivated with prolonged reaction time because the active gold species Au3+ is reduced to Au0.25 To overcome this difficulty, J. Zhang et al. studied the deactivation mechanism of AuCl3 catalyst through the AuCl3 dimer model and density functional theory (DFT).26 M. Zhu et al. developed various methods to enhance the activation and stability of Au3+ by preparing AuCl3/PPy–MWCNTs catalysts.27–29 L. Kang et al. investigated the reaction mechanism of the hydrochlorination of acetylene to C2H3Cl over MClx (M = Hg, Au, Ru; x = 2, 3) catalyst. They concluded that RuCl3 could be a good candidate catalyst based on theoretical calculations.30 Other scientists have researched the mechanisms, active sites, and deactivation.31–33 Most of the reported studies are focused on metal chloride catalysts,34–38 and research on non-metallic catalysts, especially C-doped BN cages, for acetylene hydrochlorination is limited.39–43
In the present study, DFT was used to study the adsorption and dissociation of C2H2 and HCl on un-doped B12N12 and C-doped B12−nN11+nC (n = 0, 1) cages, respectively. The mechanisms of acetylene hydrochlorination to vinyl chloride catalyzed by BNC (B12−nN11+nC (n = 0, 1)) cages were examined. These investigations on the reaction mechanism of acetylene hydrochlorination on BN cages can aid the design of new environmentally benign, non-mercury catalysts and facilitate the sustainable development of the polyvinyl chloride industry.
2. Computational methods
All DFT calculations were executed using the Guassian09 program package.44 No symmetry constraints were imposed on the geometry optimization. The hybrid density functionals of Lee, Yang and Parr (B3LYP)45 with the 6-31+G** basis set were applied for all structures, including reactants, products, intermediates, and transition states. All stationary points mentioned were characterized as minima (no imaginary frequency) or transition states (one imaginary frequency) through a Hessian calculation. Intrinsic reaction coordinate (IRC)46,47 calculations were performed to confirm that the reaction links the correct products to the reactants. Transition-state structures were characterized using frequency calculations and by analyzing the vibrational modes. In all instances, only one imaginary frequency corresponding with the reaction coordinate was obtained. Basis set superposition error48 corrections evaluated by the counterpoise method were considered.Considering frontier molecular orbital (FMO) and charge-distribution analysis, we can estimate the approximate distribution of the active sites of the BNC cages, as well as the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energies of the C2H2, HCl, and BNC cages. By calculating the HOMO–LUMO energy gaps, we obtained the lowest HOMO–LUMO energy gaps and ascertained the electron donor and acceptor. We have further verified the predicted consequence of FMO analysis through charge-distribution analysis.
3. Results and discussion
3.1. Geometries of reactants and adsorption onto BNC cages
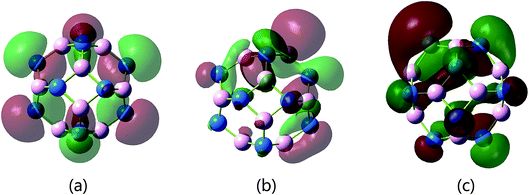
By separately substituting one C atom for a B or N atom in B12N12, B11N12C and B12N11C were obtained. As shown in the HOMO in Fig. 2(b), electrons accumulated around the C1 atom in B11N12C, as well as in B12N11C (Fig. 2(c)). The charges of the C1 atom in B11N12C and B12N11C were −0.812 and −0.023, respectively. As a result, C-doping lengthened the adjacent C1–N3 bond from 1.439 Å to 1.418 Å (Fig. 1(b)) and the C1–B3 bond to 1.546 Å in B12N11C (Fig. 1(c)), which made the C1 atom appear slightly embossed. The Mulliken charges of the C1 atom were −0.81 in B11N12C and −0.023 in B12N11C. The C1 site was more active than any other site in the cages.
| Ead = Eadsorption state − (Ereactant + Ecatalyst) | (1) |
Table 2 shows the adsorption energies of C2H2 and HCl on the nanocages. The adsorption energies of C2H2 and HCl onto B12N12 were very low, suggesting that C2H2 and HCl cannot stably adsorb onto the B12N12 cage and be efficiently activated by catalyst.
Table 1 and Fig. 3 list the orbital energies of the HOMOs and LUMOs of C2H2, HCl, B12N12, B11N12C, and B12N11C, as well as their energy gaps. The energy gaps of the HOMO–LUMO (C2H2 → BNC) were smaller than those of the HOMO–LUMO (BNC → C2H2), indicating that C2H2 was an electron donor. In contrast to C2H2, the energy gaps of HOMO–LUMO (HCl → BNC) were larger than those of HOMO–LUMO (BNC → HCl), indicating that HCl was an electron acceptor. The energy gaps represented the ability of electrons to transfer from C2H2/HCl to the BNC cages. Fig. 3 clearly shows that the energy gaps of HOMO–LUMO (C2H2 → BNC) were smaller than those of HOMO–LUMO (HCl → BNC). Thus, we can predict that acetylene was better adsorbed onto the BNC cages than HCl, as verified by comparing adsorption energies.
| HOMO–LUMO | HOMO–LUMO | HOMO–LUMO | HOMO–LUMO | HOMO–LUMO | |||
|---|---|---|---|---|---|---|---|
| (BNC → C2H2) | (BNC → HCl) | (C2H2/HCl → B12N12) | (C2H2/HCl → B11N12C) | (C2H2/HCl → B12N11C) | |||
| HOMO | LUMO | C2H2 | HCl | B12N12 | B11N12C | B12N11C | |
| C2H2 | −8.08 | 0.35 | 6.84 | 5.81 | 7.62 | ||
| HCl | −9.19 | −0.42 | 7.95 | 6.92 | 8.73 | ||
| B12N12 | −7.95 | −1.24 | 8.30 | 7.53 | |||
| B11N12C | −5.76 | −2.27 | 6.11 | 5.34 | |||
| B12N11C | −7.43 | −0.46 | 7.78 | 7.01 | |||
 | ||
| Fig. 3 The energy gaps of HOMO–LUMO between adsorbates (C2H2 and HCl) and BNC clusters are illustrate in this figure for intuitive understanding. Further detail data is provides in Table 1. | ||
The corresponding adsorption energies of C2H2 and HCl adsorbed onto BNC cages are displayed in Fig. 4. Besides, Table 2 lists the optimal adsorption energies of C2H2 and HCl onto the BNC cages. We can clearly observe that C2H2 was more easily adsorbed onto BNC than HCl, which confirmed the previous prediction obtained by the HOMO–LUMO energy gaps. These cages primarily adsorbed C2H2 to form BNC-C2H2 complexes, which subsequently adsorbed HCl because of the considerable difference in the adsorption energies of C2H2 and HCl. This conclusion was the same as that drawn from the HOMO–LUMO energy gap analysis.
| B12N12 | B11N12C | B12N11C | |
|---|---|---|---|
| C2H2 | −0.79 | −25.70 | −27.58 |
| HCl | −0.50 | −3.06 | −0.16 |
To identify the most favorable adsorption configurations on the B11N12C and B12N11C cages, a C2H2 molecule was originally placed in different sites on the surface of the nanocages with different directions. We obtained the three most stable single-component adsorption configurations (A, B, and C), as shown in Fig. 5. A and B corresponds to a C2H2 molecule adsorbed onto a B11N12C cage, and C was corresponds to a C2H2 molecule adsorbed onto a B12N11C cage. Fig. 5(A and B) show that the C2H2 adsorbed onto the B11N12C cage in a different way, i.e., in the trans configuration for A and in the cis configuration for B. This subtle distinction considerably influenced the mechanism, as elaborated in the next part. Given that the π molecular orbital of C2H2 transferred to the BNC cages, the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond became a double bond, and the bond length increased from 1.208 Å to 1.312, 1.312, and 1.317 Å. A C–C single bond formed between the C2 atom of acetylene and the C1 atom of the BNC cage, and the bond lengths were 1.513, 1.506, and 1.517 Å, respectively. More distortions were observed in the angles of the C2H2 molecule, which revealed that C2H2 molecular was well-activated. These results further demonstrated that C-doped B12−nN11+nC (n = 0, 1) fullerenes had considerable sorption capacity and that the C1 site had high activity.
C triple bond became a double bond, and the bond length increased from 1.208 Å to 1.312, 1.312, and 1.317 Å. A C–C single bond formed between the C2 atom of acetylene and the C1 atom of the BNC cage, and the bond lengths were 1.513, 1.506, and 1.517 Å, respectively. More distortions were observed in the angles of the C2H2 molecule, which revealed that C2H2 molecular was well-activated. These results further demonstrated that C-doped B12−nN11+nC (n = 0, 1) fullerenes had considerable sorption capacity and that the C1 site had high activity.
3.2. Reaction mechanisms of acetylene hydrochlorination over BNC cages
The possible reaction pathways were systematically examined to gain a better understanding of the reaction mechanism of B11N12C and B12N11C catalyzing the reaction of acetylene hydrochlorination. Fig. 6 shows the reaction pathways starting from HCl and C2H2 co-adsorption structures (Fig. 5(A, B, and C)) denoted as R1, R2, and R3, respectively. The structures of the various stationary points located on the potential energy surface are depicted in Fig. 7–9, along with the values of the most relevant geometrical parameters. | ||
| Fig. 7 Optimized structures of stationary points for reaction channel R1. The blue arrow presents the direction of vibration of H1 atom. All distances are in Å and angles are in °. | ||
The HCl molecule subsequently adsorbed onto the C2H2–B11N12C complex to form a co-adsorption configuration. Considering the changes in the bond length and adsorption energy, HCl was nearly unactivated in the co-adsorption state. The co-adsorption structure can be converted into the de-adsorption structure through the transition state Ts1 (Fig. 7). In the Ts1 state, the HCl molecule shifted to the other side of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and closer to the C
C double bond and closer to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. Meanwhile, the bond length of H–Cl increased from 1.306 Å to 1.877 Å compared with the co-adsorption state. The H1 atom approached the C2 atom, and the distance between them decreased to 1.355 Å. This finding proved that the HCl molecule completely dissociated and was activated in the Ts1 state. Only one imaginary frequency (−1090.30 cm−1) was obtained from the vibrational analysis of the Ts1 structure. This frequency was associated with the stretching movement of the H1 atom. To achieve the Ts1 state, a climb over the energy barrier of 36.08 kcal mol−1 (the activation energy of acetylene hydrochlorination) was required. This step was the rate-limiting step. To evaluate the catalyst activity of B11N12C in acetylene hydrochlorination, we compared the energy barrier in the rate-limiting step on B11N12C with other calculated non-metal catalysts (Table 3). The results showed that B11N12C performed well.
C double bond. Meanwhile, the bond length of H–Cl increased from 1.306 Å to 1.877 Å compared with the co-adsorption state. The H1 atom approached the C2 atom, and the distance between them decreased to 1.355 Å. This finding proved that the HCl molecule completely dissociated and was activated in the Ts1 state. Only one imaginary frequency (−1090.30 cm−1) was obtained from the vibrational analysis of the Ts1 structure. This frequency was associated with the stretching movement of the H1 atom. To achieve the Ts1 state, a climb over the energy barrier of 36.08 kcal mol−1 (the activation energy of acetylene hydrochlorination) was required. This step was the rate-limiting step. To evaluate the catalyst activity of B11N12C in acetylene hydrochlorination, we compared the energy barrier in the rate-limiting step on B11N12C with other calculated non-metal catalysts (Table 3). The results showed that B11N12C performed well.
To gain a better understanding of the reaction mechanism, an IRC calculation was performed to confirm that the transition state structure linked the co-adsorption complex to the de-adsorption complex. No further intermediates were involved in the reactions. In the de-adsorption state, the target product C2H3Cl molecule adsorbed onto B11N12C cages to form adsorption complexes. The bond length of the calculated C2–C3 was 1.488 Å, longer than the 1.329 Å of the free C2H3Cl molecule. The final step was the desorption of C2H3Cl molecules, and the desorption energy was 25.41 kcal mol−1, which was equal to the adsorption energy of the C2H3Cl molecule onto the B11N12C cage.
The structure of co-adsorption can change to Ims1 with transition state Ts1. In this structure, the H1–Cl bond was substantially lengthened and induced the elimination of thee HCl molecule. The H1–Cl bond length increased from 1.302 Å to 1.517 Å compared with the co-adsorption state in R2. A significant change in the coordination of the HCl molecule also occurred. The Cl atom approached the C2 site in R2, and the distance between them decreased from 3.647 to 1.988 Å compared with the co-adsorption state, indicating that HCl was completely activated. We observed an interesting phenomenon about the Ts1 state (Fig. 9) in R3. The H–Cl bond was not only broken but also nearly adsorbed in parallel onto the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and formed a dihedral angle of 0.883 between the H1–Cl and C2–C3 atoms. At the same time, the H–Cl bond length increased from 1.306 Å to 1.552 Å, but the other critical bonds, such as C1–C2 and C2–C3, underwent only very slight changes.
C bond and formed a dihedral angle of 0.883 between the H1–Cl and C2–C3 atoms. At the same time, the H–Cl bond length increased from 1.306 Å to 1.552 Å, but the other critical bonds, such as C1–C2 and C2–C3, underwent only very slight changes.
 | ||
| Fig. 9 Optimized structures of stationary points for reaction channel R3. The blue arrow presents the direction of vibration of H1 atom. All distances are in Å and angles are in °. | ||
The only imaginary frequency (−2480 cm−1 in R2 and −1780.75 cm−1 in R3) was obtained from the vibrational analysis of the Ts1 structure. This phenomenon was associated with the stretching movement of the H1 atom. To achieve the Ts1 state, a climb over the energy barriers of 49.63 and 41.41 kcal mol−1 (the activation energy of acetylene hydrochlorination) was required. This step was the rate-controlling step for both pathways.
To gain a better understanding of the reaction mechanism, an IRC calculation was performed for co-adsorption conversion to Ims1. The IRC calculation confirmed that the Ts1 state linked the co-adsorption and Ims1 states. In the Ims1 state, a C3–Cl bond was formed by the attraction of the C3 atom. Fig. 8 and 9 show that the HCl molecule dissociated in Ts1, and the Cl atom and the nearest C3 atom had opposite charges. The charges of the Cl atom were 0.089 in R2 and 0.129 in R3. The C3 atom had opposite charges of −0.163 in R2 and −0.070 in R3. The interaction between the opposite charges caused the chlorine atom to transfer from HCl to the C3 site of the C2H2–B11N12C complex to form a C3–Cl bond in the Ims1 state. The distance between the C3 and Cl atom decreased from 1.988 Å to 1.745 Å in R2 and 1.753 Å in R3 compared with the Ts1 state.
The intermediate state Ims1 can generate a de-adsorption product with transition state Ts2. In the Ts2 state of both pathways, the H1 atom that separated from the HCl molecule almost perpendicularly approached the C2 site. The distance between the C2 and H1 atoms was 2.169 Å in R2 and 2.09 Å in R3. The only imaginary frequency (475.15 cm−1 in R2 and −459.88 cm−1 in R3) was obtained from the vibrational analysis of the Ts2 structure, which was associated with the stretching movement of the H1 atom. To obtain the Ts2 state of R2, the Ims1 state required climbing over an energy barrier of only 1.4 kcal mol−1. The corresponding energy barrier of R3 was 1.34 kcal mol−1. Thus, the H1 atom was easily attracted by the C2H2Cl–B12−nN11+nC (n = 0, 1) complex.
IRC calculation was performed again to confirm that the Ts2 state can convert into the de-adsorption state, in which the target product C2H3Cl molecule adsorbed onto B12−nN11+nC (n = 0, 1) cages to form adsorption complexes. In spite of the different adsorption configurations in R1 and R2, the same de-adsorption configuration was formed. In the de-adsorption state of R3, the bond length of the calculated C2–C3 was 1.489 Å, longer than 1.329 Å in the free C2H3Cl molecule. The final step was the desorption of the C2H3Cl molecule, and the desorption energy in R3 was 23.98 kcal mol−1, which was equal to the adsorption energy of the C2H3Cl molecule adsorbed onto the B12N11C cage.
4. Conclusions
According to our DFT calculations at the B3LYP/6-31+G** level, the doping of C atoms on the BN cages can significantly intensify the adsorption ability of the C2H2 molecule compared with the un-doped BN cages. The first step of the reaction was C2H2 adsorption, and the rate-limiting step was the dissociation of the HCl molecule in the TS1 state. In addition, we found that the trans-adsorption of the C2H2 molecule on the B11N12C cage was more favorable for acetylene hydrochlorination than cis-adsorption. Trans-adsorption can efficiently reduce the activation barrier and shorten the reaction pathway. The energy barrier for H1–Cl scission in pathway R1 was much lower than those in R2 and R3, suggesting that acetylene hydrochlorination much more easily occurred than in R1 and R3. Our investigations indicated that B11N12C performed well among non-metal catalysts and can be a candidate catalyst for acetylene hydrochlorination. We hope that our results can be useful for designing and developing novel nonmetallic catalysts.Acknowledgements
We gratefully acknowledge the National Natural Science Fundation of China (NSFC, Grant No. 11304208) and the Science and Technology Fund Projects of Shihezi University (no. 2014ZRKXJQ03).References
- H. Kroto, R. E. Smalley and J. Heath, Nature, 1985, 318, 162–163 CrossRef CAS PubMed.
- T. Guo, C. Jin and R. E. Smalley, J. Phys. Chem., 1991, 95, 4948–4950 CrossRef CAS.
- F. Jensen and H. Toftlund, Chem. Phys. Lett., 1993, 201, 89–96 CrossRef CAS.
- D. L. Strout, J. Phys. Chem. A, 2000, 104, 3364–3366 CrossRef CAS.
- H. Wu, X. Xu, H. Jiao, F. Zhang and J. Jia, Chin. Sci. Bull., 2003, 48, 1102 CrossRef CAS.
- T. Oku, A. Nishiwaki and I. Narita, Sci. Technol. Adv. Mater., 2004, 5, 635–638 CrossRef CAS PubMed.
- M. D. Esrafili and R. Nurazar, Superlattices Microstruct., 2014, 67, 54–60 CrossRef CAS PubMed.
- T. Oku, M. Kuno and I. Narita, J. Phys. Chem. Solids, 2004, 65, 549–552 CrossRef CAS PubMed.
- M. D. Esrafili and R. Nurazar, Comput. Mater. Sci., 2014, 92, 172–177 CrossRef CAS PubMed.
- T. Oku, Energies, 2015, 8, 319–337 CrossRef CAS PubMed.
- A. Soltania, M. T. Baei, E. T. Lemeski and M. Shahini, Superlattices Microstruct., 2014, 76, 315–325 CrossRef PubMed.
- G. Seifert, P. W. Fowler, D. Mitchell, D. Porezag and T. Frauenheim, Chem. Phys. Lett., 1997, 268, 352–358 CrossRef CAS.
- W. H. Moon, M. S. Son and H. J. Hwang, Appl. Surf. Sci., 2007, 253, 7078–7081 CrossRef CAS PubMed.
- T. Oku, T. Hirano, M. Kuno, T. Kusunose, K. Niihara and K. Suganuma, Mater. Sci. Eng., B, 2000, 74, 206–217 CrossRef.
- H. Wu, X. Fan and J. Kuo, Int. J. Hydrogen Energy, 2012, 37, 14336–14342 CrossRef CAS PubMed.
- H. Wu, X. Fan, J. Kuo and W. Deng, Chem. Commun., 2010, 46, 883–885 RSC.
- G. J. Hutchings and D. T. Grady, Appl. Catal., 1985, 16, 411–415 CrossRef CAS.
- G. J. Hutchings and D. T. Grady, Appl. Catal., 1985, 17, 155–160 CrossRef CAS.
- G. J. Hutchings, J. Catal., 1985, 96, 292–295 CrossRef CAS.
- B. Nkosi, N. J. Coville and G. J. Hutchings, J. Chem. Soc., Chem. Commun., 1988, 71–72 RSC.
- X. Wei, F. Wei, W. Qian, G. Luo, H. Shi and Y. Jin, Chin. J. Process Eng., 2008, 6, 034 Search PubMed.
- Q. Song, S. Wang, B. Shen and J. Zhao, Pet. Sci. Technol., 2010, 28, 1825–1833 CrossRef CAS PubMed.
- U. Dzhemilev, O. Vostrikova and G. Tolstikov, AE Shilov, Gordon and Breach, New York, 1986, vol. 2 Search PubMed.
- S. A. Mitchenko, E. V. Khomutov, A. A. Shubin and Y. M. Shul'ga, J. Mol. Catal. A: Chem., 2004, 212, 345–352 CrossRef CAS PubMed.
- B. Nkosi, M. D. Adams, N. J. Coville and G. J. Hutchings, J. Catal., 1991, 128, 378–386 CrossRef CAS.
- J. Zhang, Z. He, W. Li and Y. Han, RSC Adv., 2012, 2, 4814–4821 RSC.
- X. Li, M. Zhu and B. Dai, Appl. Catal., B, 2013, 142–143, 234–240 CrossRef CAS PubMed.
- C. Huang, M. Zhu, L. Kang and B. Dai, Catal. Commun., 2014, 54, 61–65 CrossRef CAS PubMed.
- C. Huang, M. Zhu, L. Kang, X. Li and B. Dai, Chem. Eng. J., 2014, 242, 69–75 CrossRef CAS PubMed.
- M. Zhu, L. Kang, Y. Su, S. Zhang and B. Dai, Can. J. Chem., 2013, 91, 120–125 CrossRef CAS.
- M. Conte, A. F. Carley, C. Heirene, D. J. Willock, P. Johnston, A. A. Herzing, C. J. Kiely and G. J. Hutchings, J. Catal., 2007, 250, 231–239 CrossRef CAS PubMed.
- K. Zhou, W. Wang, Z. Zhao, G. Luo, J. T. Miller, M. S. Wong and F. Wei, ACS Catal., 2014, 4, 3112–3116 CrossRef CAS.
- M. Conte, A. F. Carley, G. Attard, A. A. Herzing, C. J. Kiely and G. J. Hutchings, J. Catal., 2008, 257, 190–198 CrossRef CAS PubMed.
- J. Ma, S. Wang and B. Shen, React. Kinet., Mech. Catal., 2013, 110, 177–186 CrossRef CAS.
- S. Wang, B. Shen and Q. Song, Catal. Lett., 2009, 134, 102–109 CrossRef.
- J. Zhao, X. Cheng, L. Wang, R. Ren, J. Zeng, H. Yang and B. Shen, Catal. Lett., 2014, 144, 2191–2197 CrossRef CAS PubMed.
- J. Zhao, J. Zeng, X. Cheng, L. Wang, H. Yang and B. Shen, RSC Adv., 2015, 5, 16727–16734 RSC.
- K. Zhou, J. Jia, C. Li, H. Xu, J. Zhou, G. Luo and F. Wei, Green Chem., 2015, 17, 356–364 RSC.
- X. Li, Y. Wang, L. Kang, M. Zhu and B. Dai, J. Catal., 2014, 311, 288–294 CrossRef CAS PubMed.
- B. Dai, K. Chen, Y. Wang, L. Kang and M. Zhu, ACS Catal., 2015, 5, 2541–2547 CrossRef CAS.
- X. Wang, B. Dai, Y. Wang and F. Yu, ChemCatChem, 2014, 6, 2339–2344 CrossRef CAS PubMed.
- K. Zhou, B. Li, Q. Zhang, J. Q. Huang, G. L. Tian, J. C. Jia, M. Q. Zhao, G. H. Luo, D. S. Su and F. Wei, ChemSusChem, 2014, 7, 723–728 CrossRef CAS PubMed.
- K. Zhou, J. Si, J. Jia, J. Huang, J. Zhou, G. Luo and F. Wei, RSC Adv., 2014, 4, 7766–7769 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, H. P. H. X. Li, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian Inc., Wallingford CT, 2010 Search PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, J. Chem. Phys., 1989, 90, 2154–2161 CrossRef CAS PubMed.
- C. Gonzalez and H. B. Schlegel, J. Phys. Chem., 1990, 94, 5523–5527 CrossRef CAS.
- F. B. van Duijneveldt, J. G. van Duijneveldt-van de Rijdt and J. H. van Lenthe, Chem. Rev., 1994, 94, 1873–1885 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra08266h |
| This journal is © The Royal Society of Chemistry 2015 |