Bistable polymer-dispersed cholesteric liquid crystal thin film enabled by a stepwise polymerization
Abstract
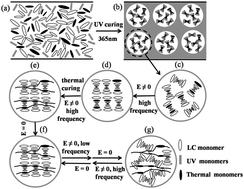
In this paper, by using a novel polymer network and an ion-doped cholesteric liquid crystal (ChLC) with negative dielectric anisotropy, we successfully fabricated a bistable polymer-dispersed liquid crystal (PDLC) thin film by the optically-induced polymerization of acrylate monomers followed by the thermal curing of epoxy groups. The stepwise processes not only enable an enhancement in the mechanical properties of PDLC films but also a stabilization of cholesteric textures by polymer networks. The bistable memory effects came from the anchoring properties of polymer networks formed within the microdroplets of PDLCs. The devices prepared from the films can be reversibly switched between a transparent state and an opaque state by an electric field. When the electric field is removed, the states are retained. Therefore, the devices are highly energy efficient.

 Please wait while we load your content...
Please wait while we load your content...