Vegetation identification based on characteristics of fluorescence spectral spatial distribution
Abstract
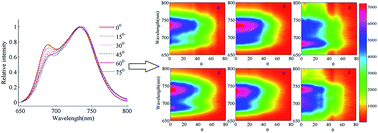
To differentiate and analyze plant types and species, a spectral identification approach is proposed founded on the characteristics of fluorescence spectral spatial distribution. Pseudo-color images of fluorescence spectral spatial distribution outperforming steady-state fluorescence spectra are constructed to serve as individual fingerprints for plant types and species, which can be utilized to accurately discriminate different plant types and species especially the different species of the same family. The introduced method provides a more reliable and stabilized means for identifying and analyzing plant species in the fields of vegetation-ecology and remote sensing. Stability and reliability are validated by using the spatial distribution of fluorescence spectra measurements of paddy rice and Dracaena sanderiana at two different incident angles of excitation light source in an additional experiment.

 Please wait while we load your content...
Please wait while we load your content...