Investigation on the aggregation behavior of photo-responsive system composed of 1-hexadecyl-3-methylimidazolium bromide and 2-methoxycinnamic acid
Abstract
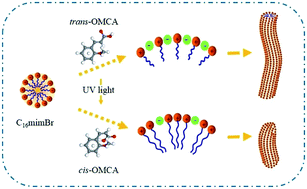
A novel fluid system composed of 2-methoxycinnamic acid (trans-OMCA) and 1-hexadecyl-3-methylimidazolium bromide (C16mimBr) in an aqueous solution was investigated. The compounds trans-OMCA and C16mimBr in an aqueous solution can self-assemble and form viscoelastic worm-like micelles. The concentrations of trans-OMCA and C16mimBr have a significant influence on the rheological properties of the system. The samples were characterized by rheological measurements. The structural isomerization of trans-to-cis for trans-OMCA occurred after UV light irradiation. The transformation of the system after UV light irradiation was determined by UV-vis absorption spectroscopy, rheological measurement and cryo-TEM observation. Surface tension measurements were carried out to investigate the role of trans-OMCA and UV light in C16mimBr aqueous solution. Critical aggregation concentration (cac), effectiveness of surface tension reduction (Πcac), maximum excess surface concentration (Γmax) and minimum area occupied per surfactant molecule (as) were investigated. Critical packing parameter was introduced to express the mechanism of aggregation behavior transition.

 Please wait while we load your content...
Please wait while we load your content...