DOI:
10.1039/C5RA08094K
(Paper)
RSC Adv., 2015,
5, 57671-57677
Electrochemical sensors of octylphenol based on molecularly imprinted poly(3,4-ethylenedioxythiophene) and poly(3,4-ethylenedioxythiophene–gold nanoparticles)†
Received
2nd May 2015
, Accepted 24th June 2015
First published on 24th June 2015
Abstract
Two novel molecularly imprinted polymer (MIP)–graphene nanoribbon (GNR) composite film coated glassy carbon electrodes (GCE) were presented. The GNRs were prepared by unzipping multiwalled carbon nanotubes through a microwave-assisted method in the presence of ionic liquid. The 4-tert-octylphenol (OP) imprinted polymers were electrochemically synthesized at the GNR modified electrodes (GNRs/GCE), using 3,4-ethylenedioxythiophene (EDOT) and gold nanoparticles (AuNPs)-captured EDOT (EDOT–Au) as monomers, and the resulting electrodes were MIPEDOT/GNRs/GCE and MIPEDOT–Au/GNRs/GCE, respectively. An EDOT–Au precursor solution was prepared by mixing EDOT and AuNPs. The imprinting process and test conditions were optimized. The resulting electrodes MIPEDOT/GNRs/GCE and MIPEDOT–Au/GNRs/GCE showed good performance when they were used for the voltammetric determination of OP due to the synergistic effect of GNRs, AuNPs and MIP. Under the optimized conditions, the peak currents of OP at the MIPEDOT/GNRs/GCE and MIPEDOT–Au/GNRs/GCE were linear with its concentration in the ranges of 0.04–8 μM and 0.02–8 μM with sensitivities of 4.87 μA μM−1 and 7.28 μA μM−1 respectively; the corresponding detection limits were 6 nM and 1 nM (S/N = 3). The MIPEDOT–Au/GNRs/GCE was more sensitive than the MIPEDOT/GNRs/GCE due to the enhancement of AuNPs. In addition, the sensors showed good selectivity to OP compared with nonimprinted electrodes. When they were applied to the electrochemical determination of OP in real samples, satisfying results were obtained.
1. Introduction
4-tert-Octylphenol (OP) is a vital environmental contaminant and it is very toxic to aquatic organisms and can probably cause significant endocrine disruption.1,2 It is a main intermediate material and degradation product of octylphenol polyethoxylate, accounting for about 20% of commercial alkylphenol polyethoxylate, only behind nonylphenol polyethoxylate.3 Since 2000, OP was included in the list of priority hazardous substances by Directive 2000/60/EC.4 Hence, the monitoring of OP in the environment is of great significance. Generally, alkylphenols are detected by liquid chromatography5–8 and gas chromatography.9–11 Although electrochemical methods were also exploited for the determination of alkylphenols, most of them aimed at nonylphenol12–14 and just a few concerned other alkylphenols. As examples, Ana-Maria Gurban et al.15 developed an alkylphenol biosensor by entrapping horseradish peroxidase in a nanocomposite gel of single-walled carbon nanotubes and 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid. The linear detection range and the detection limit for OP were 5.5–97.7 μM and 0.4 μM, respectively. In another paper,16 they prepared a xenoestrogen sensor by direct precipitation of manganese oxide onto a screen-printed carbon electrode. The linear detection range was 14–616 μM and the detection limit was 0.7 μM for OP. The two sensors suffered from low sensitivity and a high limit of detection and could hardly fulfil the monitoring of OP, which is usually at low concentration levels in the environment and can adversely affect organisms even at μg L−1 levels.16 Zheng et al.17 developed a simple and sensitive electroanalytical method for the determination of 4-n-octylphenol, an isomer of OP, based on multi-walled carbon nanotubes (MWCNTs) modified glassy carbon electrode (GCE). Wan et al.18 fabricated an electrochemical sensor for the determination of OP by electrochemical synthesis of poly(L-lysine) film on a carbon nanotubes modified GCE. Those sensors showed high sensitivity due to the enhancement of modification materials, but they suffered from the interference of other phenols. In the article,18 the authors outspoke that molecularly imprinted polymer (MIP) might be required for the selective determination of OP in environmental aquatic samples. But to the best of our knowledge, no such work has been reported till now.
Electropolymerization is an appealing way to prepare MIP as it is simple, fast and easy-controlled. Pyrrole and phenylenediamine easily form polymer,19 hence they are usually used as monomers for electrochemical imprinting. For examples, Chen et al.20 prepared an imprinted polypyrrole film at nickel nanoparticles–graphene modified carbon electrode for the determination of tetrabromobisphenol A. Du et al.21 fabricated a dimethoate imprinted electrode using o-phenylenediamine as electropolymerization monomer. The obtained sensors showed improved selectivity towards the target substances. However, the monomers suffer from some drawbacks. Pyrrole is somewhat monotonous in regard of structure and functional group, while poly(o-phenylenediamine) is quite delicate in organic solvent and it can be easily destroyed by elution. 3,4-Ethylenedioxythiophene (EDOT) has an sulphur atom and two ether groups, which may benefit formation of high-quality imprinted film. Moreover, poly(3,4-ethylenedioxythiophene) (PEDOT) has good conductivity and stability in both water and organic solvents. Hence, EDOT may be a promising monomer for electropolymerization imprinting. However, EDOT was seldom used as monomer in imprinting process,22,23 though PEDOT was often used for fabricating electrochemical sensors.24,25
Graphene nanoribbons (GNRs) are thin elongated strips of graphene (GN) and promising materials for the preparation of sensors. Martín et al.26 found that some phenols showed better electrochemical responses at GNRs than at MWCNTs modified GCE. In addition, gold nanoparticle (AuNP) was well applied in the fabrication of electrochemical sensors due to its high specific surface area, catalytic activity and other super performance.27–29 Many articles reported the electrochemical determination of phenols by using AuNPs-contained device.30–32
In this work, we prepared two electrochemical sensors by using GNRs as electrode modification material, EDOT or AuNPs-captured EDOT (EDOT–Au) as monomer for electrochemical imprinting. The GNRs and AuNPs were introduced to enhance the sensitivity, while MIP to improve the selectivity. The obtained sensors were applied to the detection of OP in water and urine samples.
2. Experimental
2.1. Apparatus
Linear sweep voltammetry and cyclic voltammetry experiments were performed with a CHI 440 electrochemical workstation (CH Instrument Company, Shanghai, China). A conventional three-electrode system was adopted, including a modified GCE (diameter: 2 mm) as working electrode, a Pt counter electrode, a saturated calomel electrode (SCE) or an Ag/AgCl electrode as reference electrode. The solution pH value was measured with PHS-3C pH indicator (Lei-Ci Instrument Company, Shanghai, China). Ultraviolet visible (UV-Vis) absorption spectra were recorded by a U-3900 spectrometer (Hitachi Co., Japan). The Fourier transform infrared (FT-IR) absorption spectra were recorded with a model Nexus-670 spectrometer (Nicolet, USA). Scanning electron microscope (SEM) images were obtained using a Sigma FESEM (Zeiss, Germany). GNRs, PEDOT, AuNPs and PEDOT–Au were dropped or electrodeposited on GCEs for SEM image observation. The microwave reaction was carried out in a NN-S3240WEF microwave oven (Panasonic, Japan).
2.2. Reagents
MWCNTs (diameter: 10–20 nm; length: 0.2–2 μm; purity: >95 wt%) was purchased from Xianfeng Reagent Co. Ltd (Nanjing, China). 4-tert-Octylphenol of analytical grade was supplied by J&K Scientific Ltd (Beijing, China), and its stock solution (0.010 M) was prepared with ethanol for further dilution. 1-Butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) was provided by Lanzhou Institute of Chemical Physics (Lanzhou, China) and used as received. Chloroauric acid, sodium citrate, lithium perchlorate, acetonitrile, sodium dodecyl benzene sulfonate (SDBS) and EDOT were obtained from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Other reagents used were of analytical grade and the water used was ultra-pure (UP).
2.3. Fabrication of sensors
GNRs were prepared by unzipping MWCNTs through a microwave-assisted method33 in the presence of [BMIM][BF4]. Briefly, 0.020 g MWCNTs and 0.10 g [BMIM][BF4] were mixed and ultrasonicated to obtain a homogeneous viscous solution. Subsequently, this solution was placed in a microwave oven and irradiated for 240 s at high heat mode. The resulting black solid was washed with dimethyl formamide and dried in the air. Then the material was dispersed into water to form 0.5 mg mL−1 GNR suspension with the aid of ultrasonication. AuNPs was prepared by reducing chloroauric acid with sodium citrate according to the report34 with a little modification. Briefly, 5 mL sodium citrate (20 mM) was rapidly injected into boiling aqueous solution of chloroauric acid (50 mL, 0.5 mM) under vigorous stirring. After 15 min more the burgundy solution was cooled to room temperature. Then the as-obtained AuNPs were mixed with EDOT (10 mM) to prepare EDOT–Au precursor solution.
Prior to modification, the bare GCE was polished with slurry alumina (Φ = 0.5 μm) and washed with ethanol, acetone and UP water successively, with the aid of ultrasonication, and then 8 μL GNR suspension was dropped onto its surface. After the solvent was evaporated, the obtained GNRs/GCE was immersed into acetonitrile solution for electrochemical imprinting, which contained 3 mM EDOT, 1 mM OP and 0.05 M LiClO4. The potential scan was performed between 0 V and 1.4 V (vs. Ag/AgCl) for 9 cycles at 50 mV s−1 and the resulting electrode was denoted as MIPEDOT/GNRs/GCE. At the same time, an MIPEDOT–Au/GNRs/GCE was fabricated through similar procedure, but the solution was replaced by an aqueous solution containing 4 mM EDOT–AuNPs, 1 mM OP, 0.05 M LiClO4 and 2 mM SDBS, and the potential was cycled between 0 V and 1.2 V (vs. SCE) for 12 times. After electropolymerization the electrodes were eluted with MeOH–HAc solution (v/v: 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 1 h, thus imprinted electrodes were obtained (Fig. 1). Non-imprinted electrodes (i.e. NIPEDOT/GNRs/GCE and NIPEDOT–Au/GNRs/GCE) were prepared under the same conditions but in the absence of OP.
1) for 1 h, thus imprinted electrodes were obtained (Fig. 1). Non-imprinted electrodes (i.e. NIPEDOT/GNRs/GCE and NIPEDOT–Au/GNRs/GCE) were prepared under the same conditions but in the absence of OP.
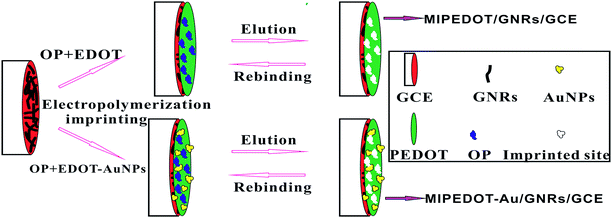 |
| | Fig. 1 The preparation routes for MIPEDOT/GNRs/GCE and MIPEDOT–Au/GNRs/GCE. | |
2.4. Electrochemical measurements
The electrode-system was immersed into a phosphate buffer solution (PBS, pH = 7.0) containing certain OP. After an open-circuit accumulation of 15 min, linear sweep voltammogram (LSV) was recorded from 0.2 V to 1.0 V at 100 mV s−1. After every measurement, the electrode was renewed by washing with MeOH–HAc solution (v/v: 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and repeating potential scan between 0.2 V and 1.0 V in a PBS (pH = 7.0) until a stable cyclic voltammogram (CV) was obtained.
1) and repeating potential scan between 0.2 V and 1.0 V in a PBS (pH = 7.0) until a stable cyclic voltammogram (CV) was obtained.
3. Results and discussion
3.1. Morphological characterization
As shown in Fig. 2, the widths of obtained GNRs (Fig. 2a) are approximately 40 nm, which is about two times as big as that of the diameter of pristine MWCNTs, and some cracks can be seen on the surface and the edge of GNRs. This demonstrates that the MWCNTs are unzipped successfully. After the potentiodynamic scans, a uniform and dense PEDOT film forms on the electrode surface (Fig. 2b). Comparing with the SEM image of AuNPs (Fig. 2c), it can be known that PEDOT–Au film is prepared successfully on the electrode surface (Fig. 2d). This is because EDOT competes with citrate and captures AuNPs due to the affinity of sulphur atom in thiophene ring. Thereafter AuNPs are anchored both in and on the PEDOT film during the electropolymerization.
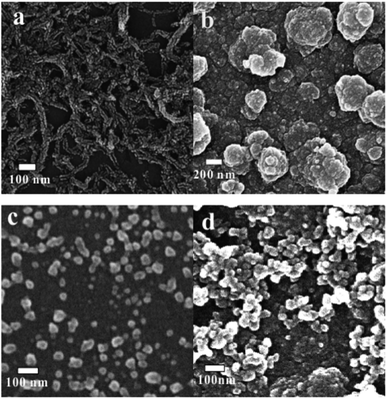 |
| | Fig. 2 SEM images of GNRs (a), PEDOT (b), AuNPs (c) and PEDOT–Au (d). | |
3.2. Electrochemical response of OP at different electrodes
As can be seen in Fig. 3, the MIPEDOT/GNR/GCE (Fig. 3c) and NIPEDOT/GNR/GCE (Fig. 3d) both exhibit more sensitive response than MIP/GCE (Fig. 3a) and NIP/GCE (Fig. 3b). This indicates that GNRs can improve the sensitivity. In addition, the peak current of OP at MIPEDOT/GNRs/GCE (Fig. 3c) is several times as large as that at NIPEDOT/GNRs/GCE (Fig. 3d). Similar phenomenon can be seen when EDOT–Au is used as monomer. The peak current of OP at the MIPEDOT–Au/GNRs/GCE (Fig. 4a) is about four times as large as that at the NIPEDOT–Au/GNRs/GCE (Fig. 4b), indicating that the MIP shows stronger affinity to OP. For comparison, Au/MIPEDOT/GNRs/GCE (Fig. 4d) and MIPEDOT/Au/GNRs/GCE (Fig. 4e) were prepared by modifying AuNPs after and before electrochemical synthesis of imprinted PEDOT film, respectively. But they did not show such sensitive electrochemical response to OP as MIPEDOT–Au/GNRs/GCE (Fig. 4a). The reason may be that through the procedures the AuNPs cover the GNRs or imprinted film, making some active area or imprinted sites unavailable. For MIPEDOT–Au/GNRs/GCE, the AuNPs are anchored closely to MIP and to some extent the imprinting occurs on the AuNPs surface, which benefits the adsorption and response of OP.
 |
| | Fig. 3 LSVs of MIP/GCE (a), NIP/GCE (b), MIPEDOT/GNRs/GCE (c) and NIPEDOT/GNRs/GCE (d) in 0.1 M PBS (pH = 7.0) containing 4 μM OP. Scan rate: 100 mV s−1; accumulation time: 15 min. | |
 |
| | Fig. 4 LSVs of MIPEDOT–Au/GNRs/GCE (a), NIPEDOT–Au/GNRs/GCE (b), MIPEDOT/GNRs/GCE (c), Au/MIPEDOT/GNRs/GCE (d) and MIPEDOT/Au/GNRs/GCE (e) in OP solution. Other conditions as in Fig. 3. | |
3.3. Optimization of conditions
3.3.1. Electrode modification. The amount of GNR suspension was optimized (Fig. S1†). The peak current of OP at MIPEDOT/GNR/GCE increased with the amount of GNR suspension rising to 8 μL, and then it kept almost unchanged. Hence 8 μL GNR suspension was adopted.
3.3.2. The composition of electropolymerization solution. As EDOT is easy to polymerize in acetonitrile and OP at millimole concentration can be readily dissolved in it, acetonitrile was used as solvent for the preparation of OP-imprinted PEDOT film. At the same time, 0.05 mM LiClO4 was chosen as the electrolyte and an Ag/AgCl was used as the reference electrode. As shown in Fig. S2a,† the electropolymerization could occur at potential over 1.2 V. Hence the high potential of 1.4 V (vs. Ag/AgCl) was adopted in potentiodynamic scan for electropolymerization. The FT-IR spectrum of the polymer was shown in Fig. S3.† The absorption band at about 1380 cm−1 in MIPEDOT was attributed to the symmetric deformation vibration of methyl group in OP molecule, which indicated that OP was successfully imprinted into PEDOT film. As for the precursor solution of EDOT–Au, acetonitrile caused severe agglomeration and precipitation of AuNPs. Hence, the MIPEDOT–Au/GNRs/GCE was prepared in aqueous solution. When EDOT and AuNPs were mixed in water, the AuNP suspension turned dusty blue from yellow. Meanwhile, the adsorption peak at about 530 nm decreased and a new peak emerged at about 660 nm (Fig. S4†). This indicated that EDOT interacted with AuNPs and their sizes increased. To facilitate the formation of imprinted PEDOT–Au film in aqueous solution, SDBS was introduced to increase the solubility of OP and lower the overpotential for polymerization.35 After adding SDBS, the suspension became limpid, and the electropolymerization could initiate at about 1.0 V (vs. SCE) (Fig. S2b†). In this case the high potential of 1.2 V (vs. SCE) was adopted for MIPEDOT–Au/GNRs/GCE preparation. Fig. S5† showed the CV of MIPEDOT–Au/GNRs/GCE in 0.05 M H2SO4, the peaks at 1.1 V and 0.85 V corresponded to the electro-oxidation and electro-reduction of AuNPs. This indicated that AuNPs were anchored to the polymer.
3.3.3. Imprinting conditions. The thickness of imprinted film was optimized by controlling the number of scan cycle for both MIPEDOT/GNRS/GCE and MIPEDOT–Au/GNRS/GCE. As could be seen in Fig. 5a, at the MIPEDOT/GNRS/GCE the peak current of OP reached the maximum value when the number of scan cycle was 9. Similarly, the number of scan cycle for MIPEDOT–Au/GNRS/GCE preparation was also optimized to be 12 (Fig. 5b), which was 3 cycles more than that for MIPEDOT/GNRS/GCE. The reason may be that it was more difficult for EDOT to form polymer in aqueous solution than in organic solvent. Another reason was that the high potential adopted for MIPEDOT–Au/GNRS/GCE preparation was lower than that for MIPEDOT–Au/GNRS/GCE. The ratio of monomer to template does influence the performance of the resulting imprinted sensor. Herein batch experiments were carried out to find the best ratio (Fig. 6), in which the template concentration (1 mM) was kept constant, while the monomer concentration was changed. When the ratio was too low, the monomer was not adequate to form enough imprinting sites; when too high monomer might induce high cross-linking degree and lead to poor permeability of the resulting film, hampering the diffusion of target to the recognition sites. In this case, the maximum peak currents were obtained at the ratios of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for MIPEDOT/GNRs/GCE (Fig. 6a) and MIPEDOT–Au/GNRs/GCE (Fig. 6b), respectively. The corresponding ratios were adopted in following electropolymerization.
1 for MIPEDOT/GNRs/GCE (Fig. 6a) and MIPEDOT–Au/GNRs/GCE (Fig. 6b), respectively. The corresponding ratios were adopted in following electropolymerization.
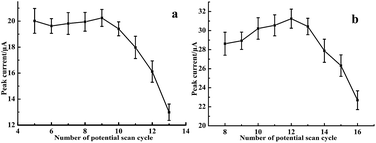 |
| | Fig. 5 Influence of the number of potential scan cycle on the peak currents of resulting MIPEDOT/GNRs/GCE (a) and MIPEDOT–Au/GNRs/GCE (b). Other conditions as in Fig. 3. | |
 |
| | Fig. 6 Influence of monomer-to-template ratio on the peak currents of resulting MIPEDOT/GNRs/GCE (a) and MIPEDOT–Au/GNRs/GCE (b). Other conditions as in Fig. 3. | |
3.3.4. Test conditions. As presented in Fig. S6,† the peak current of 4 μM OP at the MIPEDOT/GNRs/GCE increased with prolonging accumulation time and it kept almost unchanged after 15 min, meaning that the saturated adsorption was achieved. Herein 15 min was adopted for open-circuit accumulation. As 15 min was long enough for OP to reach the adsorption equilibrium, the accumulation time was also adopted for the MIPEDOT–Au/GNRs/GCE. The solution pH was also optimized for measurement and the peak current reached its max value around pH 7.0. Hence, pH 7.0 PBS was selected (data not shown) for both sensors.
3.4. Calibration curve
Fig. 7 showed the LSVs of OP at MIPEDOT/GNRs/GCE and MIPEDOT–Au/GNRs/GCE under the optimized experimental conditions. The peak current increased with OP concentration increasing. Furthermore, the peak current of OP on the two imprinted sensors and its concentration presented fine linear relationship in a certain concentration range. Comprehensive data of the two sensors were shown in Table 1. It could be seen that the sensitivity and limit of detection (LOD) of MIPEDOT–Au/GNRs/GCE were superior to those of MIPEDOT/GNRs/GCE. Compared with some other electrodes reported,9–11 the two sensors had wider linear ranges and lower detection limits.
 |
| | Fig. 7 LSVs of OP at MIPEDOT/GNRs/GCE (a) and MIPEDOT–Au/GNRs/GCE (b). Insets: the corresponding calibration curves. Other conditions as in Fig. 3. | |
Table 1 Comparison of different electrodes for OP determination
| Electrodes |
Linear range (μM) |
LOD (μM) (S/N = 3) |
Sensitivity (μA μM−1) |
References |
| Horseradish peroxidase. Single-wall carbon nanotubes. Ionic liquid. Screen-printed electrode. Poly(L-lysine). |
| MIPEDOT/GNRs/GCE |
0.04–8 |
0.006 |
4.87 |
This work |
| MIPEDOT–AuNPs/GNRs/GCE |
0.02–8 |
0.001 |
7.28 |
This work |
| HRPa–SWCNTsb–ILc–SPEd |
5.5–97.7 |
1.1 |
— |
14 |
| MnO2/SPE |
14–616 |
0.7 |
— |
15 |
| MWCNTs/GCE |
0.05–50 |
0.015 |
— |
16 |
| PLe/CNTs/GCE |
0.0065–0.02 |
0.0005 |
— |
17 |
3.5. Selectivity, reproducibility and stability of the sensors
To evaluate the selectivity of the sensors, some foreign compounds such as 2,4-dichlorophenol, nonylphenol, bisphenol A, tetrabromobisphenol A and paracetamol, were also tested at the concentration of 4 μM. These compounds have same electroactive group and similar structure to OP and often coexist with it in the environment. As shown in Fig. 8, although undergoing the same process of electro-oxidation, the peak current of 4 μM OP at the two imprinted sensors was several times bigger than that of other compounds, indicating that the two imprinted sensors had some selectivity. Although π–π interaction and hydrogen bonds could occur between the structural analogues and the OP-imprinted polymers, the adsorption capacity for foreign compounds was much weaker than that for OP. Hence, they showed weaker response than OP at the sensors. This result revealed the recognition effect of MIPEDOT and MIPEDOT–Au, which depended on the size, shape and functional group of template. As for GNRs/GCE, NIPEDOT–Au/GNRs/GCE and NIPEDOT/GNRs/GCE, the five foreign compounds showed similar response as OP did, which indicated that GNRs/GCE and nonimprinted electrodes had poor selectivity compared with imprinted sensors. It also could be seen that the response of OP at the NIPEDOT–Au/GNRs/GCE and NIPEDOT/GNRs/GCE was obviously smaller than at GNRs/GCE. This was because the dense nonimprinted polymer film hampered the access of OP toward electrode surface to oxide.
 |
| | Fig. 8 Comparison of the peak currents of 4 μM OP, DCP, NP, BPA, TBBPA, PC at MIPEDOT–Au/GNRs/GCE (a), MIPEDOT/GNRs/GCE (b), GNRs/GCE (c), NIPEDOT–Au/GNRs/GCE (d) and NIPEDOT/GNRs/GCE (e). DCP: 2,4-dichlorophenol; NP: nonyl phenol; BPA: bisphenol A; TBBPA: tetrabromobisphenol A; PC: paracetamol. Other conditions as in Fig. 3. | |
To check the repeatability of the sensors, a 4 μM OP solution was determined for five times using a sensor and the relative standard deviation (RSD) of the peak current was calculated to be 4.1% for MIPEDOT/GNRs/GCE and 5.3% for MIPEDOT–Au/GNRs/GCE, respectively. A 4 μM OP solution was also detected with five different sensors prepared by the same way and RSDs of 6.7% (for MIPEDOT/GNRs/GCE) and 6.3% (for MIPEDOT–Au/GNRs/GCE) were obtained. When the MIPEDOT/GNRs/GCE and MIPEDOT–Au/GNRs/GCE were stored in a refrigerator at 4 °C for one month, the peak currents retained 88% and 84% of their initial values. These indicated that they had good reproducibility and stability.
3.6. Application
Since OP is widely used in the manufacture of plastic packing materials and agriculture chemicals, it might be found in the environmental water, bottled water and even body fluid of human. To evaluate the practical feasibility of the proposed sensors, river water, bottled mineral water and urine were detected. For the determination, 9 mL samples were diluted to 10 mL with 1 M PBS (pH = 7.0). But no OP was detected in the samples. Standard OP solutions were added to the samples to estimate the recovery. The data were shown in Table 2 and the recoveries were 97–111% (for MIPEDOT/GNRs/GCE) and 93–110% (for MIPEDOT–Au/GNRs/GCE), which demonstrated the accuracy of the sensors.
Table 2 Results of detection of OP in water and urine samples
| Samples |
Added (μM) |
MIPEDOT/GNRs/GCE |
MIPEDOT–Au/GNRs/GCE |
| Found (μM) |
Recovery (%) |
RSD (%) |
Found (μM) |
Recovery (%) |
RSD (%) |
| River water |
0.20 |
0.212 |
106 |
6.2 |
0.212 |
106 |
6.8 |
| 2.00 |
2.10 |
105 |
4.8 |
2.06 |
103 |
5.1 |
| Bottled water |
0.20 |
0.194 |
97 |
5.9 |
0.220 |
110 |
6.3 |
| 2.00 |
2.02 |
101 |
3.5 |
1.86 |
93 |
3.2 |
| Urine |
0.20 |
0.222 |
111 |
6.5 |
0.218 |
109 |
6.2 |
| 2.00 |
2.14 |
107 |
5.1 |
2.10 |
105 |
4.7 |
4. Conclusions
In this work, GNR was prepared and it could enhance the sensitivity of OP sensors. EDOT was a good monomer for the electrochemical imprinting and it could yield high-quality imprinted films in both aqueous solution and organic solution. In addition, anchored AuNPs could also improve the property of sensor. The as-prepared sensors presented high sensitivity, stability and selectivity in the voltammetric determination of OP. This work provided an effective way to improve the performance of MIP-based electrochemical sensors.
Acknowledgements
The authors appreciate the financial support of the National Natural Science Foundation of China (Grant No. 21277105).
References
- P. M. Nagarnaik and B. Boulanger, Chemosphere, 2011, 85, 854–860 CrossRef CAS PubMed.
- M. J. Lopez-Espinosa, C. Freire, J. P. Arrebola, N. Navea, J. Taoufiki, M. F. Fernandez, O. Ballesteros, R. Prada and N. Olea, Chemosphere, 2009, 76, 847–852 CrossRef CAS PubMed.
- A. Priac, N. Morin-Crini, C. Druart, S. Gavoille, C. Bradu, C. Lagarrigue, G. Torri, P. Winterton and G. Crini, Arabian J. Chem., 2014 DOI:10.1016/j.arabjc.2014.05.011.
- A. G. Asimakopoulos, N. S. Thomaidis and M. A. Koupparis, Toxicol. Lett., 2012, 210, 141–154 CrossRef CAS PubMed.
- E. Ferrer, E. Santoni, S. Vittori, G. Font, J. Mañes and G. Sagratini, Food Chem., 2011, 126, 360–367 CrossRef CAS PubMed.
- M. Kawaguchi, S. Takahashi, F. Seshimo, N. Sakui, N. Okanouchi, R. Ito, K. Inoue, Y. Yoshimura, S. Izumi, T. Makino and H. Nakazawa, J. Chromatogr. A, 2004, 1046, 83–88 CrossRef CAS.
- T. Benijts, W. Lambert and A. De Leenheer, Anal. Chem., 2003, 76, 704–711 CrossRef PubMed.
- R. Romero-Cano, D. Kassuha, J. Peris-Vicente, P. Roca-Genoves, S. Carda-Broch and J. Esteve-Romero, Analyst, 2015, 140, 1739–1746 RSC.
- R. Liu, J. L. Zhou and A. Wilding, J. Chromatogr. A, 2004, 1022, 179–189 CrossRef CAS PubMed.
- S. Berkner, G. Streck and R. Herrmann, Chemosphere, 2004, 54, 575–584 CrossRef CAS.
- M. Kawaguchi, K. Inoue, N. Sakui, R. Ito, S. Izumi, T. Makino, N. Okanouchi and H. Nakazawa, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 799, 119–125 CrossRef CAS PubMed.
- G. A. Evtugyn, S. A. Eremin, R. P. Shaljamova, A. R. Ismagilova and H. C. Budnikov, Biosens. Bioelectron., 2006, 22, 56–62 CrossRef CAS PubMed.
- L. Zeng, A. Zhang, X. Zhu, C. Zhang, Y. Liang and J. Nan, J. Electroanal. Chem., 2013, 703, 153–157 CrossRef CAS PubMed.
- J. Huang, X. Zhang, S. Liu, Q. Lin, X. He, X. Xing, W. Lian and D. Tang, Sens. Actuators, B, 2011, 152, 292–298 CrossRef CAS PubMed.
- A. M. Gurban, L. Rotariu, M. Baibarac, I. Baltog and C. Bala, Talanta, 2011, 85, 2007–2013 CrossRef CAS PubMed.
- A.-M. Gurban, D. Burtan, L. Rotariu and C. Bala, Sens. Actuators, B, 2015, 210, 273–280 CrossRef CAS PubMed.
- Q. Zheng, P. Yang, H. Xu, J. Liu and L. Jin, J. Environ. Sci., 2012, 24, 1717–1722 CrossRef CAS.
- Q. Wan, P. Yang, H. Cai, H. Song and N. Yang, J. Electroanal. Chem., 2013, 689, 252–256 CrossRef CAS PubMed.
- S. Liu, B. Yu and T. Zhang, J. Mater. Chem. A, 2013, 1, 13314–13320 CAS.
- H. Chen, Z. Zhang, R. Cai, W. Rao and F. Long, Electrochim. Acta, 2014, 117, 385–392 CrossRef CAS PubMed.
- D. Du, S. Chen, J. Cai, Y. Tao, H. Tu and A. Zhang, Electrochim. Acta, 2008, 53, 6589–6595 CrossRef CAS PubMed.
- C. Weng, W. Yeh, K. Ho and G. Lee, Sens. Actuators, B, 2007, 121, 576–582 CrossRef CAS PubMed.
- J. Orozco, A. Cortes, G. Cheng, S. Sattayasamitsathit, W. Gao, X. Feng, Y. Shen and J. Wang, J. Am. Chem. Soc., 2013, 135, 5336–5339 CrossRef CAS PubMed.
- G. Sheng, G. Xu, S. Xu, S. Wang and X. Luo, RSC Adv., 2015, 5, 20741–20746 RSC.
- S. Liu, J. Tian, L. Wang, Y. Luo and X. Sun, Analyst, 2011, 136, 4898–4902 RSC.
- A. Martín, J. Hernández-Ferrer, L. Vázquez, M. Teresa Martínez and A. Escarpa, RSC Adv., 2014, 4, 132 RSC.
- S. Guo and E. Wang, Anal. Chim. Acta, 2007, 598, 181–192 CrossRef CAS PubMed.
- J. M. Pingarrón, P. Yáñez-Sedeño and A. González-Cortés, Electrochim. Acta, 2008, 53, 5848–5866 CrossRef PubMed.
- M. Ozsoz, A. Erdem, K. Kerman, D. Ozkan, B. Tugrul, N. Topcuoglu, H. Ekren and M. Taylan, Anal. Chem., 2003, 75, 2181–2187 CrossRef CAS PubMed.
- V. Carralero, M. L. Mena, A. Gonzalez-Cortes, P. Yanez-Sedeno and J. M. Pingarron, Biosens. Bioelectron., 2006, 22, 730–736 CrossRef CAS PubMed.
- W. Song, D. W. Li, Y. T. Li, Y. Li and Y. T. Long, Biosens. Bioelectron., 2011, 26, 3181–3186 CrossRef CAS PubMed.
- J. Huang, X. Zhang, S. Liu, Q. Lin, X. He, X. Xing and W. Lian, J. Appl. Electrochem., 2011, 41, 1323–1328 CrossRef CAS PubMed.
- S. Vadahanambi, J.-H. Jung, R. Kumar, H.-J. Kim and I.-K. Oh, Carbon, 2013, 53, 391–398 CrossRef CAS PubMed.
- S. H. Liu and M. Y. Han, Adv. Funct. Mater., 2005, 15, 961–967 CrossRef CAS PubMed.
- X. Du and Z. Wang, Electrochim. Acta, 2003, 48, 1713–1717 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra08094k |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 1 h, thus imprinted electrodes were obtained (Fig. 1). Non-imprinted electrodes (i.e. NIPEDOT/GNRs/GCE and NIPEDOT–Au/GNRs/GCE) were prepared under the same conditions but in the absence of OP.
1) for 1 h, thus imprinted electrodes were obtained (Fig. 1). Non-imprinted electrodes (i.e. NIPEDOT/GNRs/GCE and NIPEDOT–Au/GNRs/GCE) were prepared under the same conditions but in the absence of OP.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and repeating potential scan between 0.2 V and 1.0 V in a PBS (pH = 7.0) until a stable cyclic voltammogram (CV) was obtained.
1) and repeating potential scan between 0.2 V and 1.0 V in a PBS (pH = 7.0) until a stable cyclic voltammogram (CV) was obtained.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for MIPEDOT/GNRs/GCE (Fig. 6a) and MIPEDOT–Au/GNRs/GCE (Fig. 6b), respectively. The corresponding ratios were adopted in following electropolymerization.
1 for MIPEDOT/GNRs/GCE (Fig. 6a) and MIPEDOT–Au/GNRs/GCE (Fig. 6b), respectively. The corresponding ratios were adopted in following electropolymerization.






