Tuning the antifouling property of PVDF ultrafiltration membrane with surface anchored polyelectrolyte complexes for sewage treatment
Abstract
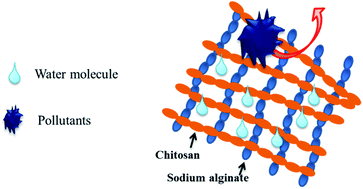
The polyelectrolyte complex (PEC) layer fabricated by chitosan and sodium alginate was anchored on a membrane surface though plasma treatment and layer by layer self-assembly to improve the antifouling properties of the PVDF membrane. The interface properties of modified PVDF membranes were investigated and the results indicated the presence of the PEC layer was conducive to enhance the hydrophilicity and screening ability of the PVDF ultrafiltration membrane due to the hydrophilic crosslinking structure. Furthermore, the fouling resistance including anti-adsorption ability and dynamic antifouling ability for pollutant filtration of modified PVDF membranes were significantly improved due to the anchored PEC layer. For the CS–SA-3 membrane with an assembly number of 3, the adsorption mass of BSA on the membrane surface was only 4 μg cm−2 and the FRR-W values increased to 89%, 99% and 98% for the three typical pollutants of bovine serum albumin, sodium alginate and humic acid, respectively. It was demonstrated that the PEC layer could be used as an antifouling material to improve the antifouling ability of hydrophobic membranes though decreasing the reversible fouling. This article also aimed to provide a simple method to fabricate the antifouling interface.

 Please wait while we load your content...
Please wait while we load your content...