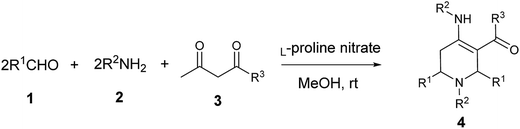
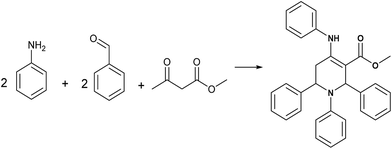
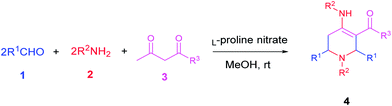
L-Proline nitrate: a recyclable and green catalyst for the synthesis of highly functionalized piperidines†
Nikita R. Agrawal‡
a,
Sandeep P. Bahekara,
Prashant B. Sarodea,
Sanjio S. Zadeb and
Hemant S. Chandak*a
aDepartment of Chemistry, G. S. Science, Arts and Commerce College, Khamgaon 444303, India. E-mail: chemants@gmail.com; Fax: +91-7263-253844
bDepartment of Chemical Sciences, Indian Institute of Science Education and Research (IISER), Mohanpur, Kolkata, 741246, India
First published on 18th May 2015
Abstract
The synthesis of highly functionalized piperidines has been strategically accessed via organo-catalytic three components (in situ five components) reaction of an amine, aldehyde and 1,3-dicarbonyl compound. This imine based multi-component reaction was realized using fully green L-proline nitrate as recyclable room temperature ionic liquid. Recycling of the catalyst was possible up to five runs without loss of catalyst activity. Smaller E-factor (0.255) and process mass intensity (PMI = 3.35), high atom-economy (AE = 89.5%) and reaction mass efficiency (RME = 79.66%) demonstrates the higher environmental compatibility and sustainability of this protocol. DFT calculations showed that the L-proline catalyzed reaction proceeds by three pathways; (i) via proline enamine pathway, (ii) proline mediated aniline enamine pathway or (iii) the pathway involving iminium activation of the aldehyde to provide the Knoevenagel product.
Introduction
In the context of sustainability, ionic liquids (ILs) have attracted great attention from the chemists in recent years.1,2 Commonly used ionic liquids, based on imidazolium cations and fluorinated anions are synthetic chemicals and therefore are not as green as desired. This demands the development of bio-degradable ILs replacing the synthetic quaternary nitrogen cations such as alkylammonium, dialkylimidazolium and pyridinium ions by bio-renewable ions. Use of bio-renewable natural compounds as starting materials for the preparation of the ions in ILs is a promising ongoing approach.3 Amino acids which represent the natural chiral pool, offers a best choice as a source of quaternary nitrogen cation. Among the various amino acids, proline plays a vital role in iminium and enamine catalysis.4Piperidine and its derivatives bestowed supreme importance in the heterocyclic as well as pharmaceutical arena.5 Many natural products6,7 possessing piperidine scaffold interestingly exhibit anti-hypertensive,8 anti-bacterial,9 anti-convulsant and anti-inflammatory activities.10 Boehm et al. in 1943 reported the first multicomponent reaction (MCR) between and amine, aldehyde and 1,3-dicarbonyl to synthesize functionalised piperidines.11 Apart from some Lewis and Brönsted acid catalysts,12 a few organocatalysts which include wet picric acid,13 p-toluenesulfonic acid,14 bromodimethylsulfonium bromide,15 tetrabutylammonium tribromide (TBATB),16 and thiourea oxide17 have been reported for this MCR. Mishra and co-workers have reported L-proline and trifluoroacetic acid (TFA) as dual catalytic system for this MCR.18 However, L-proline and TFA as dual catalytic system suffer from major drawbacks such as catalyst loadings (20% of each), longer reaction time, tiresome isolation and purification procedure and non-recyclability of the catalyst. Recently, Shaterian and Azizi used imidazolium and guanidinium acidic ionic liquid for the synthesis of functionalized piperidines.19 Although recyclability and shorter reaction time makes their protocol superior than the others but the use of chemicals like imidazole and guanidine as a source of quaternary nitrogen is still a matter of serious concern which deprives the green essence of the protocol.
To the best of our knowledge, there is only one report for the use of L-proline nitrate IL as catalyst. Kou et al. used it as a catalyst and solvent for Diels–Alder reaction.20 Despite of having advantageous properties, the catalytic potential of L-proline nitrate has not been utilized yet by the synthetic organic chemists. In this context, we evaluated the catalytic efficiency of L-proline based acidic IL in the MCR involving Aza-Diels–Alder cycloaddition of imine to enamine. We achieved this pseudo five component MCR using 10 mol% of L-proline nitrate ionic liquid as a fully green catalyst in methanol at ambient temperature leading to the formation of highly functionalised piperidines (Scheme 1).
Results and discussion
Mechanistically, multicomponent synthesis of functionalized piperidines involves Aza Diels–Alder cycloaddition of Knovenagel adduct (C) and imine (D) (Scheme 2).In order to investigate the role of proline in this reaction, DFT study (at B3LYP/631G(d)) was performed on the key reaction steps. We have considered computationally the pathways of formation of tautomer of diene (C). In these reaction pathways, we have found that the addition of enamine to benzaldehyde is the rate determining step (RDS). As solvent (methanol) supposed to play important role on the mechanism, solvent effects of methanol were taken into account using polar continuum model (PCM) calculations on the optimized geometries. A transition state for the formation of enamine from methylacetoacteate with aniline is compared to that with L-proline. The activation barrier for the formation of enamine with L-proline is 6.2 kcal mol−1 which is 7.2 kcal mol−1 lower than that with aniline. Again L-proline is considered to catalyse this reaction by two pathways either via proline enamine pathway or proline mediated aniline enamine pathway as shown in the Fig. 1 and S1.† Both the pathways have less activation energy (8.9 and 11.9 kcal mol−1 for pathway (b) and proline mediated aniline enamine pathway) for the rate determining steps than that of in absence of proline (21.7 kcal mol−1 for pathway (a)). Thus L-proline catalyzes the reaction either by forming enamine with acetoacetate ester in the first step (pathway (b)) or by facilitating the proton transfer through its molecular framework (Fig. S1†). In addition to this, we have considered the pathway involving iminium activation of the aldehyde to provide the Knoevenagel product (Fig. S2†). However, this pathway has similar energy profile compared to that of pathway (a).
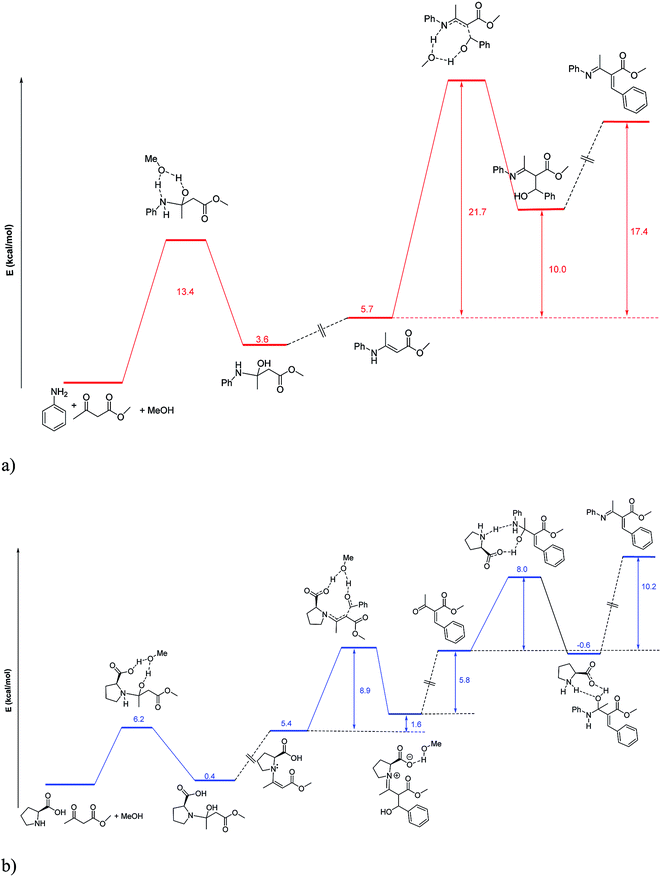 | ||
| Fig. 1 DFT investigation of formation of enamines and Knoevenagel adducts. (a) Energetics of uncatalysed reaction. (b) Energetics of the reaction via L-proline enamine pathway. | ||
These considerations from DFT calculation and the role of proline in iminium and enamine catalysis, prompted us to employ a fully green L-proline based acidic IL for the synthesis of functionalised piperidines. L-Proline nitrate was prepared by treating L-proline with nitric acid using the reported procedure.20 Initially, the reaction of benzaldehyde, aniline and methyl acetoacetate was screened with various potential catalysts (Table 1). In presence of L-proline nitrate, the reaction proceeds smoothly giving corresponding highly diversified piperidines in good yields. Thus, encouraged with these results, we focused on the optimization reaction conditions as shown in Table 1. The finest result was accomplished in the presence of 10 mol% of the catalyst in methanol medium at ambient temperature (Table 1, entry 6). Optimal amount of catalyst is advisable since lower (5 mol%, Table 1, entry 8) as well as higher amounts (upto 30 mol%) did not put any desirable impact on the yield of products even after the longer reaction time. Additionally, the effect of different solvents such as ethanol and CH3CN was also investigated, and was found to be insignificant (Table 1, entry 5 and 7). Methanol was found to be the best choice as a solvent, used directly without rigorous drying. Methanol can dissolve all starting materials and the product formed is nearly insoluble in it. This allows us to isolate the desired product straightforwardly by filtration.
| Entry | Catalyst | Condition | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a General reaction conditions: methylacetoacetate (1.75 mmol), aniline (3.5 mmol), benzaldehyde (3.5 mmol), rt.b Isolated yield. | ||||
| 1 | None | Methanol | 24 | Trace |
| 2 | Succinic acid (10 mol%) | Methanol | 30 | 37 |
| 3 | L-Ascorbic acid (10 mol%) | Methanol | 24 | 40 |
| 4 | L-Proline + TFA (20 mol%) | Acetonitrile | 17 | 70 (ref. 18) |
| 5 | L-Proline nitrate (10 mol%) | Ethanol | 8 | 69 |
| 6 | L-Proline nitrate (10 mol%) | Methanol | 8 | 90 |
| 7 | L-Proline nitrate (10 mol%) | Acetonitrile | 8 | 79 |
| 8 | L-Proline nitrate (5 mol%) | Methanol | 24 | 59 |
The green chemistry metrics like E-factor, atom-economy, process mass intensity and reaction mass efficiency concepts have been widely embraced by the chemical industry and the academic community.21 This optimised protocol has favourable green chemistry metrics such as smaller E-factor (0.255) and process mass intensity (PMI = 3.35); higher atom-economy (AE = 89.5%) and reaction mass efficiency (RME = 79.66%) (see ESI† for details of the calculations).
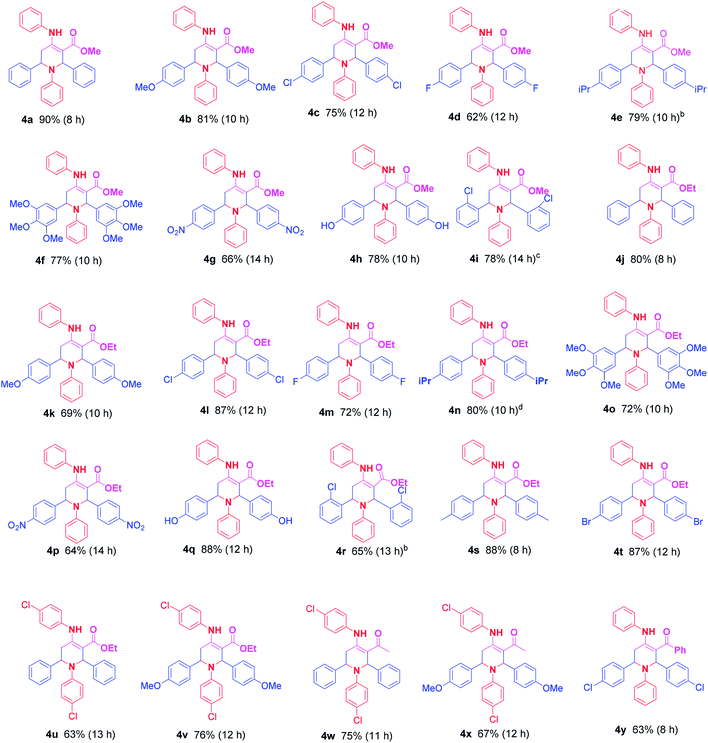
The generality and scope of this optimized protocol was tested with various aldehydes, anilines and 1,3-dicarbonyls. The outputs are summarized in Table 2. In all cases we achieved high yields of the products. Among the various aldehydes, aromatic aldehydes with electron donating as well as electron withdrawing group reacts smoothly giving high yields of functionalised piperidines. As evident from Table 2, aromatic aldehydes with nitro- and methoxy substituent did offer only moderate yield of the product.22 Aromatic amines with chloro substitution also entered smoothly in the play and gives good to high yields of functionalised piperidines (Table 2, 4u–4x). The R3 group of 1,3-dicarbonyl compounds has little or no effect on reaction. This observation is in line with the previous report.15
a General reaction conditions: 1,3-dicarbonyl (1.75 mmol), amine (3.5 mmol), aldehyde (3.5 mmol), L-proline nitrate (10 mol%), methanol (0.5 mL), rt.b Diastereotropic ratio = syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti: 10 anti: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91 (as evident from 1H NMR).c Diastereotropic ratio = syn 91 (as evident from 1H NMR).c Diastereotropic ratio = syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti: 13 anti: 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 (as evident from 1H NMR).d Diastereotropic ratio = syn 87 (as evident from 1H NMR).d Diastereotropic ratio = syn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anti: 09 anti: 09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 91 (as evident from 1H NMR). 91 (as evident from 1H NMR). |
|---|
 |
Synthesized functionalised piperidines are characterized by IR, 1H-NMR, and 13C-NMR spectra. The IR spectrum of the compounds perfectly indicates the conjugation of the carbonyl and olefinic groups, exhibiting the absorption band at around 1648–1573 cm−1. The ESIMS (mass spectra) of the compounds showed molecular ion peaks at their respective m/z.
In the 1H NMR spectrum, the proton attached to C-2 of the piperidine ring was observed as singlet at around δ 6.01–6.46 ppm or it come into view along with the multiplets of aromatic protons ranging from δ 6.35 to 7.50 ppm. The methylene protons of carbethoxy group appeared as multiplet at around δ 4.15–4.41 ppm. The only exchangeable secondary amine proton (NH) connected at C-4 appeared as broad singlet at around δ 10.29 ppm. The multiplet pertaining to the methylene protons, triplet of methyl group of carbethoxy moiety and signals of secondary amino group have been observed at two different chemical shifts in a few cases.23 The 13C NMR spectra showed requisite number of distinct resonances in agreement with the proposed structure. The anti stereochemistry of the product was also confirmed by single crystal X-ray analysis of the product 4o (Fig. 2).
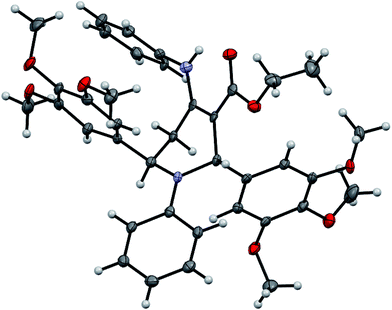 | ||
| Fig. 2 ORTEP diagram of compound 4o.† | ||
The ease with which product was isolated from the reaction mixture prompted us to think about the recovery of the catalyst from residue. To recover the catalyst, initially methanol was removed under reduced pressure. Proline nitrate IL is soluble in water (or ethanol or acetone) and is insoluble in ethyl acetate (or chloroform or benzene). The careful examination of the solubility properties of proline nitrate suggests two ways to recover the catalyst – (1) to dissolve all the components of residue except catalyst in ethyl acetate or chloroform and (2) to dissolve catalyst in water and remove the rest of the residue by filtration. The first method was ruled out as washing with the volatile organic compound creates waste stream. We preferred the second possibility, and taking the advantage of the solubility of the IL in water, the residue was washed with a little amount of water to get an aqueous solution of the catalyst as a filtrate. Water from the filtrate was removed under reduced pressure and the last traces of water were removed by forming azeotrope with a very little amount of toluene to get the catalyst which was then available for the next run.
Conclusion
Scope, optimization, and application of the L-proline nitrate catalyzed pseudo five component reaction leading to the formation of functionalized piperidines have been described herein. Of the several important improvements, a few are: (1) it is the first L-proline nitrate ionic liquid catalysed synthesis of functionalised piperidines. (2) The reaction has operational simplicity and uses an inexpensive, nontoxic and biocompatible catalyst. (3) Simple purification of product without the use of column chromatography. (4) The formation of highly distereoselective products with generally high yield. (5) Higher atom economy (AE) and reaction mass efficiency (RME). (6) Lower E factor and process mass intensity (PMI).DFT calculations showed that the L-proline can act as catalyst in three catalytic pathways which proceed either via (i) formation of proline enamine or (ii) formation of proline mediated aniline enamine or (iii) proline mediated iminium activation of the aldehyde to provide the Knoevenagel product. These three pathways are significantly lower in energetics than that of uncatalyzed reaction. We have developed green protocol using a ‘fully green’ L-proline nitrate ionic liquid for the synthesis of functionalised piperidines. Additional attributes of this procedure over previous processes are minimal usage of organic solvent, simple work-up procedure, recyclability and biocompatibility of the catalyst. The green chemistry metrics calculations reveals smaller E-factor (0.255) and process mass intensity (PMI = 3.35), high atom-economy (AE = 89.5%) and reaction mass efficiency (RME = 79.66%), approves the higher environmental compatibility and sustainability of this protocol.
Experimental
General procedure for the synthesis of functionalised piperidines
A mixture of 1,3-dicarbonyl (1.75 mmol), amine (3.5 mmol), aldehyde (3.5 mmol) and L-proline nitrate (31.2 mg, 0.175 mmol) in MeOH (0.5 mL) was stirred at room temperature for an appropriate time (Table 2). After completion of the reaction, as indicated by TLC, solid obtained was filtered under suction to get product of sufficient purity. To recover the catalyst, initially methanol was removed under reduced pressure and residue was washed with a little quantity of water to get an aqueous solution of the catalyst as a filtrate. Water from the filtrate was removed under reduced pressure and the last traces of water were removed by forming azeotrope with a very little amount of toluene to get the catalyst which was then available for the next run.Computational details
All calculations were performed using Gaussian 09 programme.24 The geometries involved in the key reaction steps were fully optimized by using a hybrid25 Becke three-parameter exchange density functional with the LYP correlation functional (B3LYP)26 and the 6-31G(d) basis set (B3LYP/6-31G(d) method). Transition states were located by using the TS routine. At the same level frequency calculations were performed for all stationary points to differentiate them as minima or saddle points. The energies of optimized structures were corrected using unscaled zero-point vibrational energies (ZPVEs). Gas phase values were corrected by using single-point calculations on gas phase optimized geometries for methanol as a solvent with the Polarized Continuum Model (PCM) at the same level of theory.27Acknowledgements
Authors are thankful to UGC New Delhi, India (F. no. 41-335/2012 (SR) dt.13.07.2012) for the financial support.References
- B. Kirchner and B. Clare, Ionic liquids, Springer Science & Business Media, 2009 Search PubMed.
- P. Wasserscheid and T. Welton, Ionic liquids in synthesis, Wiley Online Library, 2008 Search PubMed.
- S. T. Handy, Chem.–Eur. J., 2003, 9, 2938–2944 CrossRef CAS.
- (a) B. List, Synlett, 2001, 2001, 1675–1686 CrossRef; (b) B. List, Tetrahedron, 2002, 58, 5573–5590 CrossRef CAS.
- (a) N. G. Kozlov and A. P. Kadutskii, Tetrahedron Lett., 2008, 49, 4560–4562 CrossRef CAS; (b) S. G. Gladstone, W. G. Earley, J. K. Acker and G. S. Martin, Tetrahedron Lett., 2009, 50, 3813–3816 CrossRef CAS; (c) S. G. Davies, P. M. Roberts and A. D. Smith, Org. Biomol. Chem., 2007, 5, 1405–1415 RSC; (d) S. V. Karthikeyan, S. Perumal and K. K. Balasubramanian, Tetrahedron Lett., 2007, 48, 6133–6136 CrossRef CAS; (e) J. Esquivias, R. G. Arrayás and J. C. Carretero, J. Am. Chem. Soc., 2007, 129, 1480–1481 CrossRef CAS PubMed; (f) X.-F. Zhu, J. Lan and O. Kwon, J. Am. Chem. Soc., 2003, 125, 4716–4717 CrossRef CAS PubMed; (g) A. Takemiya and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 6042–6043 CrossRef CAS PubMed; (h) M. Amat, O. Bassas, M. A. Pericàs, M. Pastó and J. Bosch, Chem. Commun., 2005, 1327–1329 RSC; (i) R. Martín, C. Murruzzu, M. A. Pericas and A. Riera, J. Org. Chem., 2005, 70, 2325–2328 CrossRef PubMed; (j) P. Li, L.-J. Liu and J.-T. Liu, Org. Biomol. Chem., 2011, 9, 74–77 RSC; (k) D. P. Harrison, M. Sabat, W. H. Myers and W. D. Harman, J. Am. Chem. Soc., 2010, 132, 17282–17295 CrossRef CAS PubMed.
- C. Viegas, V. d. S. Bolzani, M. Furlan, E. J. Barreiro, M. C. M. Young, D. Tomazela and M. N. Eberlin, J. Nat. Prod., 2004, 67, 908–910 CrossRef CAS PubMed.
- P. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, Wiley, New York, 2nd edn, 2002, pp. 307–316 Search PubMed.
- S. Petit, J. Nallet, M. Guillard, J. Dreux, R. Chermat, M. Poncelet, C. Bulach, P. Simon, C. Fontaine and M. Barthelmebs, Eur. J. Med. Chem., 1991, 26, 19–32 CrossRef CAS.
- Y. Zhou, V. E. Gregor, B. K. Ayida, G. C. Winters, Z. Sun, D. Murphy, G. Haley, D. Bailey, J. M. Froelich and S. Fish, Bioorg. Med. Chem. Lett., 2007, 17, 1206–1210 CrossRef CAS PubMed.
- H. Bin, A. M. Crider and J. P. Stables, Eur. J. Med. Chem., 2001, 36, 265–286 CrossRef.
- T. Boehm and W. Stöcker, Arch. Pharm., 1943, 281, 62–77 CrossRef.
- (a) InCl3: P. A. Clarke, A. V. Zaytsev and A. C. Whitwood, Synthesis, 2008, 3530–3532 CrossRef CAS; (b) Iodine: A. T. Khan, M. M. Khan and K. K. Bannuru, Tetrahedron, 2010, 66, 7762–7772 CrossRef CAS; (c) CAN: H.-J. Wang, L.-P. Mo and Z.-H. Zhang, ACS Comb. Sci., 2011, 13, 181–185 CrossRef CAS PubMed; (d) ZrOCl2·8H2O: S. Mishra and R. Ghosh, Tetrahedron Lett., 2011, 52, 2857–2861 CrossRef CAS; (e) BF3·SiO2: R. Ramachandran, S. Jayanthi and Y. T. Jeong, Tetrahedron, 2012, 68, 363–369 CrossRef CAS; (f) Bi(NO3)3·5H2O: G. Brahmachari and S. Das, Tetrahedron Lett., 2012, 53, 1479–1484 CrossRef CAS; (g) LaCl3·7H2O: B. Umamahesh, V. Sathesh, G. Ramachandran, M. Sathishkumar and K. Sathiyanarayanan, Catal. Lett., 2012, 142, 895–900 CrossRef CAS; (h) VCl3: S. Pal, L. H. Choudhury and T. Parvin, Mol. Diversity, 2012, 16, 129–143 CrossRef CAS PubMed; (i) NiCl2·6H2O: M. R. M. Shafiee, B. H. Najafabadi and M. Ghashang, J. Chem. Res., 2012, 36, 336–339 CrossRef CAS.
- C. Mukhopadhyay, S. Rana, R. J. Butcher and A. M. Schmiedekamp, Tetrahedron Lett., 2011, 52, 5835–5840 CrossRef CAS.
- S. S. Sajadikhah, M. T. Maghsoodlou, N. Hazeri, S. M. Habibi-Khorassani and S. J. Shams-Najafi, Monatsh. Chem., 2012, 143, 939–945 CrossRef CAS.
- A. T. Khan, T. Parvin and L. H. Choudhury, J. Org. Chem., 2008, 73, 8398–8402 CrossRef CAS PubMed.
- A. T. Khan, M. Lal and M. M. Khan, Tetrahedron Lett., 2010, 51, 4419–4424 CrossRef CAS.
- S. Verma, S. Kumar, S. L. Jain and B. Sain, Org. Biomol. Chem., 2011, 9, 6943–6948 CAS.
- M. Misra, S. K. Pandey, V. P. Pandey, J. Pandey, R. Tripathi and R. P. Tripathi, Bioorg. Med. Chem., 2009, 17, 625–633 CrossRef CAS PubMed.
- H. R. Shaterian and K. Azizi, J. Mol. Liq., 2013, 180, 187–191 CrossRef CAS.
- G.-H. Tao, L. He, W.-S. Liu, L. Xu, W. Xiong, T. Wang and Y. Kou, Green Chem., 2006, 8, 639–646 RSC.
- (a) F. I. McGonagle, H. F. Sneddon, C. Jamieson and A. J. Watson, ACS Sustainable Chem. Eng., 2014, 2, 523–532 CrossRef CAS; (b) A. D. Curzons, D. J. Constable, D. N. Mortimer and V. L. Cunningham, Green Chem., 2001, 3, 1–6 RSC.
- We found that the present protocol is not suitable for the aliphatic aldehydes.
- Two signals due to secondary NH, CH2 (H5a and H5b) and CH3 were observed in case of the compound 4e, 4i and 4n. These compounds are obtained as diasteromeric mixtures.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision C.01, Gaussian Inc., Wallingford CT, 2010 Search PubMed.
- (a) R. G. Parr and R. G. P. W. Yang, Density-functional theory of atoms and molecules, Oxford university press, 1989 Search PubMed; (b) W. Koch, M. C. Holthausen and M. C. Holthausen, A chemist's guide to density functional theory, Wiley-Vch, Weinheim, 2001 CrossRef.
- (a) C. Lee, W. Yang and R. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS; (b) A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- M. Cossi, V. Barone, R. Cammi and J. Tomasi, Chem. Phys. Lett., 1996, 255, 327–335 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Calculation of green chemistry metrics and spectral characterisation of the selected compounds. CCDC 1047298. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra08022c |
| ‡ Present address: Department of Chemistry, Govt. V.I.S.H., Amravati 444602, India. |
| This journal is © The Royal Society of Chemistry 2015 |