Raising the efficiency of petrolatum deoiling process by using non-polar modifier concentrates separated from paraffin wastes to produce different petroleum products
Magdy T. Zaky*,
Nermen H. Mohamed,
Amal S. Farag and
Fathi S. Soliman
Petroleum Refining Division, Egyptian Petroleum Research Institute (EPRI), 1-Ahmed El-Zomor Street, Hai Al-Zehour, P. O. Box: 11727, Nasr City, Cairo, Egypt. E-mail: magdytadrouszaky1961@gmail.com; Fax: +20 222747433; Tel: +20 222745902
First published on 14th August 2015
Abstract
Deoiling of crude petrolatum was enhanced by the addition of 1 wt% of non-polar modifier concentrates separated from slack wax waste and compared with pure n-alkane mixtures of (C20 + C22) and (C24 + C26). The data revealed that 1 wt% of the separated (C20 + C22) n-alkane mixture is the preferable modifier to improve the deoiling process of crude petrolatum. X-ray diffraction patterns and SEM photographs showed that the addition of 1 wt% of non-polar modifier concentrates gave hard waxes having some crystal growth and larger crystal sizes and possessing more holes than the hard waxes separated without using a modifier. Different petroleum products were produced using both of the products of the petrolatum deoiling process: the microcrystalline wax and the slop wax. Various grades of hardened ceresin were formulated by the addition of low density polyethylene to the separated microcrystalline wax. Fourteen formulated blends of petrolatum were prepared based on the microcrystalline wax and slop wax saturate with heavy and light paraffin oils, respectively. According to the standard specifications of the US Pharmacopoeia and National Formulary of petrolatum and Ultra Chemical Inc. of liquid petrolatum, the blend formulations (3–8) were classified as technical petrolatums. Two of these blends (7 & 8) were also classified as liquid petrolatums. The blend formulations (9–14) were classified as white pharmaceutical petrolatums. Meanwhile, three of these blends (12–14) were also within the limits of the standard specifications of ultrapure liquid petrolatums.
1. Introduction
Petroleum waxes are broadly defined as the waxes naturally present in various fractions of crude petroleum. Originally, they were considered as by-products in the dewaxing of lubricating and gas oils, but today they are valuable products for many industrial applications.1Petroleum waxes in their various fields of application require many different specifications. These can only partly be satisfied by suitable choice of feedstock and manufacturing processes, or even by some modifications of these processes. Petroleum waxes are used in a wide variety of applications. They are used in the paper, match, rubber, ink, electrical, dental and cosmetics industries and in household chemicals, building construction and the manufacture of metals and ceramics.
As the consumption of wax products increases, the increased profitability of wax production will depend on the improvement and modification techniques of macro- and microcrystalline waxes as base materials as well as the development of other wax products.2–4
Microcrystalline waxes are solid at room temperature, have melting points in the range of 60–93 °C and are usually produced from heavy petroleum distillates or residues or tank bottoms. Petrolatum is a general term applied to a crude microcrystalline wax containing some oil. It is a semi-solid, jelly-like material. It is a base material for the manufacture of medicinal petroleum jelly or medicinal petrolatum. Slop wax is a semi-microcrystalline wax which has a lower melting point than microcrystalline wax. It is the remainder wax obtained from the petrolatum deoiling process after the separation of the microcrystalline wax. Ceresin is a hard microcrystalline wax having a higher melting point than the microcrystalline wax itself.1
In the processes of manufacturing and refining of petroleum waxes (solvent dewaxing and deoiling, respectively), crystallization takes place from solution. The precipitation of wax crystals during cooling is a function of two important processes: crystal nucleation and crystal growth. The greatest difficulties for dewaxing and deoiling petrolatum are related to the stage of filtering slurries of solid hydrocarbons that tend to form an inter-crystalline structure.5
The formation of large, multilayer, aggregated crystals requires a choice of modifiers that will rearrange the structural formations of paraffinic hydrocarbons in the required direction. Many organic additives, pour-point depressants, certain organometallic compounds and all possible macromolecular substances with a polymeric structure have been tested in the laboratory as modifiers for the structural conversion of the solid phase. Many of these additives, while they have a strong modifying effect, will lose this property when the medium is moist.6
Additives with various functional actions e.g. surfactants, have been tested as polar modifiers in the process of deoiling petrolatums. Not all of the modifiers are effective for the modification of the solid hydrocarbon crystal structure in the production of high-melting microcrystalline waxes. The improvement in the phase separation rate in filtering the solid hydrocarbon slurry and the decrease in the oil content in the microcrystalline wax depend on the length of the radical and the polarity of the modifier molecule.7 Aqueous sodium chloride was tested as an ionic modifier for deoiling petrolatum. It increased the selectivity of the highest-melting hydrocarbons but did not affect the filtration rate.8
The addition of non-polar modifiers, especially individual n-alkanes with an even number of carbon atoms in the molecule (C20–C24), to the wax deoiling solvent to improve the crystallization of solid hydrocarbons during the deoiling of petrolatum to produce microcrystalline wax was studied. The filtration rate was greatly increased and the resulting microcrystalline wax contained <0.5 wt% oil. These modifiers are highly effective in the range of 0.5–1 wt%.5,9–11 However, the application of these modifiers in industrial deoiling installations is not economical because of the high cost of pure n-alkanes. On the other hand, these hydrocarbons occur as petroleum by-products during lubricating oil manufacture. Thus, our work aims to raise the efficiency of the petrolatum deoiling process by improving the crystallization of solid hydrocarbons and accelerating the filtration rate to produce different petroleum products by using non-polar modifier concentrates of (C20 + C22) and (C24 + C26) n-alkane mixtures separated from slack wax waste.
2. Materials and methods
2.1. Materials
One appropriate crude petrolatum (petroleum waxy by-product) obtained from the Alexandria Petroleum Company was used and evaluated in this study for the deoiling process and the production of different petroleum products. Its physical and molecular type composition characteristics are presented in Table 1. Also, the deoiling process of crude petrolatum and its final products are explained and presented in Scheme 1.| Characteristics | Test method | Alexandria crude petrolatum |
|---|---|---|
| Congealing point, °C | ASTM D-938 | 66 |
| Kinematic viscosity, 98.9 °C | ASTM D-445 | 14.25 |
| Refractive index, 98.9 °C | ASTM D-1747 | 1.4478 |
| Density, 70 °C | ASTM D-1418 | 0.8262 |
| Mean molecular weight | ASTM D-2502 | 680 |
| Oil content, wt% | ASTM D-721 | 11.01 |
| Needle penetration, 25 °C | ASTM D-1321 | 93 |
| Sulfur content, wt% | ASTM D-4294 | 0.65 |
| Color | ASTM D-1500 | 4.0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Molecular type composition | ||
| Total saturates, wt% | 73.83 | |
| Total aromatics, wt% | 26.17 | |
| Mono-aromatics, wt% | 12.14 | |
| Di-aromatics, wt% | 14.03 | |
2.2. Deoiling process
Alexandria crude petrolatum was subjected to a one stage fractional crystallization (deoiling process)12–14 using butyl acetate (BA) solvent at a fixed solvent feed ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for dilution and 2
1 for dilution and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S/F, by weight) for washing at an ambient fractionating temperature of 20 °C. In this technique, the high melting components of the wax (hard wax) were precipitated while the low melting ones (soft or slop wax) remained in the solution.
1 (S/F, by weight) for washing at an ambient fractionating temperature of 20 °C. In this technique, the high melting components of the wax (hard wax) were precipitated while the low melting ones (soft or slop wax) remained in the solution.
The non-polar modifier concentrates which were prepared from light slack wax waste and added to the petrolatum deoiling solvent mixture were as follows:
(a) 0.5 to 1.5 wt% of the non-polar modifier concentrate of low carbon number atoms (C20 + C22) of the light slack wax after its adduction.
(b) 1 wt% of the non-polar modifier concentrate of low carbon number atoms (C20 + C22) of the paraffins (saturates) separated from light slack wax by liquid–solid chromatography followed by its adduction.
(c) 1 wt% of the non-polar modifier concentrate of high carbon number atoms (C24 + C26) of the hard wax separated from light slack wax after deoiling at 30 °C followed by its adduction.15
The previous non-polar modifier concentrates mentioned in a, b & c were compared with two mixtures of pure n-alkanes with an even number of carbon atoms of n-(C20 + C22) and n-(C24 + C26) in the percentage of 1 wt% based on the feed. The percentages of C20, C22, C24 and C26 in the pure mixtures of (C20 + C22) and (C24 + C26) were taken as their percentages in the prepared non-polar modifier concentrates.
2.3. Production of petroleum products
Besides the production of microcrystalline waxes, different types of petroleum products were prepared for various industrial applications based on the main product (microcrystalline wax) and the by-product (slop wax) of the deoiling process.The slop wax obtained by deoiling the crude petrolatum using 1 wt% of a non-polar modifier concentrate (C20 + C22) was subjected to liquid–solid chromatography to separate its saturates. Then, the slop wax saturate was blended with light paraffin oil (9–14 blends) in the range of 10 to 60 wt% to obtain different grades of medicinal petrolatums.
All petrolatum blends were made in the molten state with vigorous stirring to ensure homogenous blends on cooling to room temperature. The operating conditions were as follows: blending temperature = 90 °C, blending time = 30 min and stirring speed = 150 rpm.
2.4. Methods of analysis
The crude wax, the isolated hard and soft waxes, and all the produced petroleum products were physically characterized according to American Society for Testing and Materials (ASTM) standard methods.16 The standard methods for analysis were congealing point (ASTM D-938), kinematic viscosity (ASTM D-445), refractive index (ASTM D-1747), density (ASTM D-1418), mean molecular weight (ASTM D-2502), oil content (ASTM D-721), cone penetration (ASTM D-937), needle penetration (ASTM D-1321), color (ASTM D-1500) and sulfur content using an X-ray fluorescence sulfur meter (ASTM D-4294).The filtration rate was calculated using eqn (1).
| R = WF/AT | (1) |
The type of the isolated hard waxes was specified according to the TAPPI-ASTM equation to classify the waxes as macrocrystalline (paraffin), semi-microcrystalline and microcrystalline waxes. The distinction between these groups is made on the basis of viscosity. The proposal is as follows using eqn (2).
| nD210 °F = 0.0001943t + 1.3994 | (2) |
The aromatic and saturate contents of the crude petrolatum and the isolated hard and soft waxes were determined using liquid–solid column chromatography. A column with a 1.3 cm diameter and a height of 130 cm packed with activated silica gel (60–200 mesh size) was used. The column was then moistened with 100 ml of n-heptane to dissipate the heat of adsorption. A 10 gram sample of the wax dissolved in a few milliliters of n-heptane was transferred to the column. The column was then eluted with 300 ml of n-heptane followed by 200 ml of benzene and finally 100 ml of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of absolute methanol and benzene.19 Fractions of 25 ml were taken from the column, the solvent was distilled off and the refractive index of each fraction was determined. According to the refractive index data at 20 °C, the elutes were combined into saturates, mono-, di- and poly-aromatics. The saturate hydrocarbons have refractive indices of not more than 1.48. The mono-cyclic, bi-cyclic and poly-cyclic aromatics have refractive indices from 1.48 to 1.53, 1.53 to 1.59 and higher than 1.59, respectively.20,21
1 mixture of absolute methanol and benzene.19 Fractions of 25 ml were taken from the column, the solvent was distilled off and the refractive index of each fraction was determined. According to the refractive index data at 20 °C, the elutes were combined into saturates, mono-, di- and poly-aromatics. The saturate hydrocarbons have refractive indices of not more than 1.48. The mono-cyclic, bi-cyclic and poly-cyclic aromatics have refractive indices from 1.48 to 1.53, 1.53 to 1.59 and higher than 1.59, respectively.20,21
The ultraviolet absorbance of the produced formulated blends (9–14) was determined at 290 nm using a UV/VIS/NIR-spectrophotometer, JASCO, V570, USA.
2.5. X-ray diffraction
X-ray diffraction patterns were obtained to study the crystal size of the hard waxes separated using the non-polar modifiers of pure and prepared mixtures of n-alkanes. They were recorded in the range 2θ = 4–70° with a step size (2θ) of 0.02 and a scan step time (s) of 0.4, using a PAnalytical-XPert Pro, (Netherlands) with K-Alpha l radiation and equipped with a Cu source with a K-alpha 1 wavelength of 1.54056 Å. The instrument was operated at 40 kV and 40 mA.The 2θ, Full Width at Half Maximum (FWHM) and d spacing were obtained. The wax crystal size was calculated according to the Scherrer eqn (3).22 It is as follows:
I = 0.9λ/B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(2θ/2) cos(2θ/2)
| (3) |
2.6. Scanning electron microscopy (SEM)
SEM was used to observe the surface, shape and crystal size of the hard waxes separated by using the non-polar modifiers of pure and prepared mixtures of n-alkanes. The wax was coated with gold by a K550X sputter coater, then scanned using a scanning electron microscope (Quanta 250 FEG, Netherlands).3. Results and discussion
3.1. Effect of the addition of the prepared concentrate and pure mixture non-polar modifiers on the petrolatum deoiling process
The effect of the addition of the non-polar modifiers (the prepared concentrates and mixtures of pure n-alkanes (C20 + C22) & (C24 + C26)) on the petrolatum deoiling process was studied at the fractionating temperature of 20 °C, with solvent ratios of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight for dilution and washing, respectively, and using butyl acetate as solvent. The data are presented in Tables 2 and 3.
1 by weight for dilution and washing, respectively, and using butyl acetate as solvent. The data are presented in Tables 2 and 3.
| Characteristics | Hard waxes isolated | |||||
|---|---|---|---|---|---|---|
| Without modifier | By using prepared non-polar modifier concentrates | |||||
| (C20 + C22)* | (C20 + C22)** | (C24 + C26)*** | ||||
| 0.5% | 1% | 1.5% | 1% | 1% | ||
| a (C20 + C22)*: non-polar modifier concentrate prepared by adduction of light slack wax. (C20 + C22)**: non-polar modifier concentrate prepared by adduction of light slack saturates. (C24 + C26)***: non-polar modifier concentrate prepared by adduction of the hard waxes isolated from light slack wax at 30 °C. | ||||||
| Yield on crude petrolatum, wt% | 54.67 | 47.89 | 47.33 | 47.38 | 47.74 | 47.10 |
| Congealing point, °C | 74 | 76.5 | 77 | 77 | 77 | 77 |
| Kinematic viscosity at 98.9 °C, mm2 s−1 | 13.00 | 11.56 | 11.51 | 11.50 | 11.52 | 11.48 |
| Refractive index at 98.9 °C | 1.4396 | 1.4364 | 1.4364 | 1.4361 | 1.4368 | 1.4365 |
| Refractive index by TAPPI-ASTM equation | 1.4315 | 1.4324 | 1.4325 | 1.4325 | 1.4325 | 1.4325 |
| Mean molecular weight | 751 | 774 | 782 | 784 | 780 | 786 |
| Oil content, wt% | 1.16 | 0.19 | 0.16 | 0.16 | 0.16 | 0.14 |
| Needle penetration at 25 °C | 25 | 19 | 17 | 17 | 17 | 15 |
| Color | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Filtration rate, kg m−2 h−1 | 415 | 529 | 562 | 562 | 561 | 569 |
| Crystal size (X-ray diffraction) | 316.3 | 385.1 | 412.2 | 412.5 | 412.0 | 540.2 |
| Type of wax | Microcrystalline wax | |||||
| Characteristics | Hard waxes isolated | ||
|---|---|---|---|
| Without modifiers | By using 1 wt% of pure n-alkane mixtures | ||
| n-C20 + n-C22 | n-C24 + n-C26 | ||
| Yield on crude petrolatum, wt% | 54.67 | 48.33 | 47.93 |
| Congealing point, °C | 74 | 76.5 | 77 |
| Kinematic viscosity at 98.9 °C, mm2 s−1 | 13.00 | 11.53 | 11.51 |
| Refractive index at 98.9 °C | 1.4396 | 1.4373 | 1.4372 |
| Refractive index by TAPPI-ASTM equation | 1.4315 | 1.4324 | 1.4325 |
| Mean molecular weight | 751 | 775 | 782 |
| Oil content, wt% | 1.16 | 0.17 | 0.14 |
| Needle penetration at 25 °C | 25 | 17 | 16 |
| Filtration rate, kg m−2 h−1 | 415 | 560 | 565 |
| Crystal size (X-ray diffraction) | 316.3 | 411.0 | 501.1 |
| Type of wax | Microcrystalline waxes | ||
Addition of the prepared non-polar modifier concentrates (C20 + C22) & (C24 + C26) during fractional crystallization results in a higher filtration rate, congealing point and mean molecular weight and a lower yield, oil content and needle penetration of the hard waxes isolated from crude petrolatum than those separated without using a modifier. It is interesting to note from the data in Table 2 that increasing the non-polar modifier concentration (of the (C20 + C22) n-alkanes prepared from the light slack wax after adduction) from 0.5 to 1 wt% during fractional crystallization leads to a slight increase of the filtration rate, congealing point and mean molecular weight and a slight decrease of the yield, oil content and needle penetration of the hard waxes isolated from crude petrolatum. On the other hand, no improvement was observed on increasing the modifier concentration from 1 to 1.5 wt%. Also, the addition of 1 wt% of the non-polar modifier concentrate of (C20 + C22) n-alkanes prepared from the light slack wax saturate after adduction gave nearly the same filtration rate, yield and wax characteristics as the addition of 1 wt% of the (C20 + C22) n-alkane concentrate prepared from the light slack wax after adduction. Thus, from an economic point of view, the non-polar modifier concentrate of (C20 + C22) n-alkanes prepared from the light slack wax after adduction is a preferable modifier to improve the fractional crystallization of crude petrolatum at the fractionating temperature of 20 °C and at the dilution solvent ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight, using butyl acetate as solvent, by saving the step of saturate separation from light slack wax. On the other hand, the addition of 1 wt% of the non-polar modifier concentrate of the high carbon number (C24 + C26) n-alkanes prepared from the hard waxes isolated from light slack wax at 30 °C after adduction gave a higher filtration rate and lower needle penetration and oil content than both the addition of 1 wt% of the non-polar modifier concentrate of the lower carbon number (C20 + C22) n-alkanes prepared from the light slack wax and its saturates after adduction. This may be because the modifier which possesses a high carbon number leads to a high crystal growth and filtration rate and consequently good characteristics.
1 by weight, using butyl acetate as solvent, by saving the step of saturate separation from light slack wax. On the other hand, the addition of 1 wt% of the non-polar modifier concentrate of the high carbon number (C24 + C26) n-alkanes prepared from the hard waxes isolated from light slack wax at 30 °C after adduction gave a higher filtration rate and lower needle penetration and oil content than both the addition of 1 wt% of the non-polar modifier concentrate of the lower carbon number (C20 + C22) n-alkanes prepared from the light slack wax and its saturates after adduction. This may be because the modifier which possesses a high carbon number leads to a high crystal growth and filtration rate and consequently good characteristics.
Comparing the effect of addition of 1 wt% of the non-polar modifiers based on mixtures of pure alkanes (Table 3) having the same percentages of C20, C22, C24 and C26 as those in the prepared ones, it can be noticed that the addition of the non-polar modifiers based on the mixtures of pure alkanes as (C20 + C22) and (C24 + C26) during fractional crystallization leads to the same trend as the prepared ones in raising the efficiency of the deoiling process.
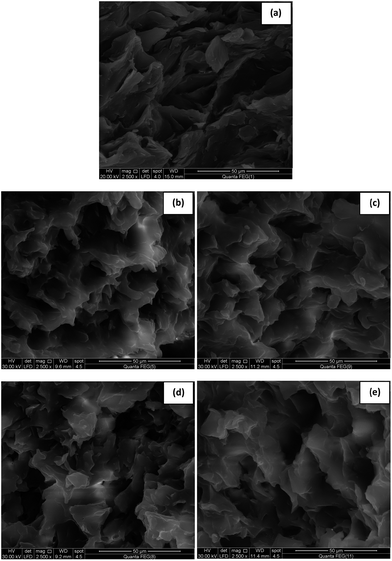
The SEM photographs of the isolated hard waxes (microcrystalline waxes) are aligned with the above findings. The crystals of the hard waxes isolated using butyl acetate with the addition of 1 wt% of non-polar modifiers – pure mixtures and prepared concentrates of (C20 + C22) & (C24 + C26) – gave higher crystal growth than the hard waxes isolated without using a modifier. Meanwhile, the crystals of the hard waxes isolated with the addition of 1 wt% of the non-polar modifiers – pure mixtures and prepared concentrates of (C24 + C26) – gave some crystal growth and possessed more holes than the others (compare Fig. 1a–e).
X-ray diffraction patterns confirm the previous results where the addition of 1 wt% of non-polar modifiers – both the pure mixtures and prepared concentrates of (C20 + C22) n-alkanes – gave nearly the same crystal sizes but these were larger than the crystal size of the hard wax isolated without using a modifier. Meanwhile, the addition of the pure mixtures and prepared concentrates of (C24 + C26) n-alkanes gave well observed crystalline patterns with larger crystal sizes (501.1 & 540.2 Å, respectively) than the formers, taking into consideration that the prepared concentrates of (C24 + C26) n-alkanes have a larger crystal size than the pure mixture of (C24 + C26) n-alkanes (Tables 2, 3 and Fig. 2). This means that the prepared concentrates of (C24 + C26) contain carbon atoms having a higher carbon number than C26, as the modifier which possesses a high carbon number leads to high crystal growth and, consequently, a large crystal size.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight and using butyl acetate as solvent, to save energy and eliminate multi-stages of the fractional crystallization process to produce hard waxes with similar characteristics. Consequently, the hard (microcrystalline) and soft (slop) waxes were separated from Alexandria crude petrolatum under these conditions.
1 by weight and using butyl acetate as solvent, to save energy and eliminate multi-stages of the fractional crystallization process to produce hard waxes with similar characteristics. Consequently, the hard (microcrystalline) and soft (slop) waxes were separated from Alexandria crude petrolatum under these conditions.3.2. Production of petroleum products
Production of different types of petroleum products was achieved by using the products (the hard wax (microcrystalline wax), the soft wax (slop wax) and its saturate) of the solvent fractional crystallization of crude petrolatum. The physical characteristics and molecular type composition of the isolated petroleum products are presented in Table 4. Hardened ceresin wax and technical petrolatums can also be produced from microcrystalline wax. In addition, medicinal and ultrapure liquid petrolatums can be produced from the soft wax (slop wax) saturate.| Characteristics | Isolated petroleum products | |||
|---|---|---|---|---|
| Microcrystalline wax | Slop wax | Slop wax saturate | Remainder oil | |
| Yield on crude petrolatum, wt% | 47.33 | 52.67 | 29.92 | 22.75 |
| Congealing point, °C | 77 | 55 | 63 | — |
| Kinematic viscosity at 98.9 °C, mm2 s−1 | 11.51 | 16.00 | 11.00 | — |
| Refractive index at 98.9 °C | 1.4364 | 1.4579 | 1.4319 | — |
| Specific gravity, 60 °C | 0.8221 | 0.8435 | 08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 308 308 |
— |
| Mean molecular weight | 782 | 557 | 682 | — |
| Oil content, wt% | 0.16 | 13.00 | 0.10 | — |
| Needle penetration at 25 °C | 17 | — | — | — |
| Cone penetration at 25 °C | — | 80 | 45 | — |
| Sulfur content, wt% | 0.23 | 1.12 | — | — |
| Color | 2.0 | 8.0 | 0.0 | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Molecular type composition | ||||
| Total saturates content, wt% | 84.20 | 56.80 | 100 | 0.00 |
| Total aromatics content, wt% | 15.80 | 43.20 | 0.0 | 100 |
| Mono-aromatics content, wt% | 15.80 | 17.24 | 0.0 | 39.91 |
| Di-aromatics content, wt% | — | 25.96 | 0.0 | 60.09 |
| Characteristics | Microcrystalline wax | Microcrystalline wax with LDPE | ||||
|---|---|---|---|---|---|---|
| 2% | 4% | 6% | 8% | 10% | ||
| Congealing point, °C | 77 | 86 | 90 | 93.5 | 97 | 99 |
| Oil content, wt% | 0.16 | 0.07 | 0.06 | 0.03 | 0.01 | 0.01 |
| Needle penetration at 25 °C | 17 | 11 | 9.5 | 8 | 7.5 | 7 |
| Kinematic viscosity, 98.9 °C, mm2 s−1 | 11.51 | 11.88 | 12.34 | 12.68 | 13.00 | 13.22 |
| Mean molecular weight | 782 | 795 | 804 | 818 | 830 | 843 |
| Color | 2.0 | 2.0 | 2.0 | 1.5 | 1.5 | 1.0 |
| Type of wax | — | Hardened ceresin | ||||
It is obvious from the data that the total aromatics are completely removed from the slop wax and consequently the total saturates reached 100 wt% upon purification treatment, i.e. aromatic free wax is produced with a lower congealing point than that of microcrystalline wax. Thus, the purified soft wax saturate can be blended with different percentages of light paraffin oil to produce different grades of medicinal petrolatum.
Six blends were made by blending the soft wax saturate with light medicinal paraffin oil (9–14 blends). The effects of the addition of light paraffin oil on the physical characteristics of the soft wax saturate are shown in Fig. 4. According to the standard specifications of the US Pharmacopoeia and National Formulary, all the formulated blends (9–14) are classified as medicinal petrolatum and can be used in the pharmaceutical, cosmetic and food fields. Meanwhile, three of the blends (12–14) are also within the limits of the standard specifications of Ultra Chemical Inc. of ultrapure liquid petrolatum as they have extra low congealing points (38–46 °C) and high penetration values (200–300 dmm at 25 °C) and can be used in formulating bar soaps, creams, lotions and hair preparations.26
Moreover, by testing the formulated blends (3–8) and (9–14), it can be concluded that all the formulated blends conform to the other specifications of petrolatum according to the definition of the US Pharmacopoeia & National Formulary (Table 6). On the other hand, the remainder oil, which contains appreciable amounts of mono- and di-aromatic constituents (39.91 & 60.09 wt%, respectively) after the separation of the saturates from the slop wax by liquid–solid chromatography (Table 4), can be used as extender oil in ink and rubber manufacturing.27
| US Pharmacopoeia & National Formulary 2011 specifications | Group 1 | Group 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| a N: neutral. | ||||||||||||
| Solubility | ||||||||||||
| Insoluble in water and ethanol. Soluble in chloroform, in ether & in petroleum spirit 40–60 °C | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Acidity | ||||||||||||
| No red or pink color to 0.1 ml of methyl orange | N | N | N | N | N | N | N | N | N | N | N | N |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Alkalinity | ||||||||||||
| The solution doesn’t acquire a pink color to one drop of phenolphthalein | N | N | N | N | N | N | N | N | N | N | N | N |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Organic acids | ||||||||||||
| For production of a sharp pink and end point not more than 400 μL of 0.1 N sodium hydroxide is required | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Color | ||||||||||||
| There is no fluorescence when the sample being held directly against a white background | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Residue on ignition | ||||||||||||
| It volatilizes without emitting an acrid odor and on ignition yields not more than 0.1% of residue and 0.05% for white petrolatum | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| Fixed oils, fats and rosin | ||||||||||||
| No oily or solid matter separates | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| UV absorbance, 290 nm | — | — | — | — | — | — | 0.096 | 0.088 | 0.079 | 0.070 | 0.061 | 0.051 |
4. Conclusions
The study shows that, from an economic point of view, the non-polar modifier concentrate (C20 + C22) n-alkanes prepared from light slack wax after adduction is a preferable modifier to improve the efficiency of crude petrolatum deoiling at the fractionating temperature of 20 °C, the dilution and washing solvent ratios of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight, respectively, and using butyl acetate as solvent to save energy and eliminate multi-stages of the fractional crystallization process to produce hard waxes in the category of microcrystalline waxes with similar characteristics.
1 by weight, respectively, and using butyl acetate as solvent to save energy and eliminate multi-stages of the fractional crystallization process to produce hard waxes in the category of microcrystalline waxes with similar characteristics.
Production of different types of petroleum products was achieved using both of the products of solvent fractional crystallization of crude petrolatum: the hard wax (microcrystalline wax) and the soft wax (slop wax). Different grades of hardened ceresin, having high congealing points, were formulated by addition of 2 to 10 wt% of low density polyethylene (LDPE) to the separated microcrystalline wax. The ceresin grades can be used in various applications such as in the paper industry, crayons, inks, coatings, electronic insulation and hot melt adhesives.
Fourteen formulated blends of petrolatum were prepared based on the produced microcrystalline wax and soft wax saturates with heavy and light paraffin oils, respectively. It can be concluded that twelve of the formulated blends (3–8) and (9–14) lie within the limits of the standard specifications of the US Pharmacopoeia and National Formulary 2011 of petrolatum and medicinal petrolatum, respectively. They can be used for industrial fields such as the lubrication of pharmaceutical machines and in the pharmaceutical, cosmetic and food fields. Meanwhile, the two formulated blends (7 & 8) and three others (12–14) are classified as liquid and ultrapure liquid petrolatums, respectively, according to the standard specifications of Ultra Chemical Inc. besides the limits of the US Pharmacopoeia and National Formulary 2011. The remainder oil produced, which contains appreciable amounts of mono- and di-aromatic constituents, can be used as extender oil in ink and rubber manufacturing.
References
- W. M. Mazee, in Modern Petroleum Technology, ed. G. D. Hobson, Applied Science Publishers Ltd., on behalf of The Institute of Petroleum, Great Britain, 1973, p. 782 Search PubMed.
- T. Miao, Shiyou Lianzhi, 1993, 24, 20–25, CA, 1994, 121(2), 13315Y.
- L. L. Kogl, Eur. Pat. Appl., 834, 618, 1998.
- M. Freund, R. Csikos, S. Keszthelyi and G. Y. Mozes, in Paraffin Products, ed. G. Y. Mozes, Elsevier Scientific Publishing Company, Hungary, 1982 Search PubMed.
- L. P. Kazakova, S. I. Kolesnikov, E. I. Vyboichenko and M. V. Mosidze, Chem. Technol. Fuels Oils, 1986, 22, 538–541 CrossRef.
- P. A. Zolotarev and R. G. Nigmatullin, Chem. Technol. Fuels Oils, 1994, 30, 418–422 CrossRef.
- L. P. Kazakova, A. A. Gundyrev, M. E. Fesenko and T. I. Sochevko, Chem. Technol. Fuels Oils, 1990, 26, 177–179 CrossRef.
- R. G. Nigmatullin, P. A. Zolotarev, G. G. Telyashev and A. K. Mukhamed’yanova, Chem. Technol. Fuels Oils, 1995, 31, 206–209 CrossRef.
- L. P. Kazakova, E. I. Vyboichenko, A. A. Gundyrev, L. P. Zubanova and M. D. Pakhomov, Chem. Technol. Fuels Oils, 1988, 24, 388–390 CrossRef.
- L. P. Kazakova, E. I. Vyboichenko, A. A. Gundyrev and L. P. Zubanova, Chem. Technol. Fuels Oils, 1989, 25, 26–28 CrossRef.
- M. T. Zaky, N. H. Mohamed, A. S. Farag and F. S. Soliman, Chem. Eng. Res. Des., 2015, 96, 130–137 CrossRef CAS PubMed.
- M. T. Zaky, N. H. Mohamed and A. S. Farag, Fuel Process. Technol., 2007, 88, 913–920 CrossRef CAS PubMed.
- N. H. Mohamed, M. T. Zaky, A. S. Farag and A. F. M. Fahmy, Pet. Sci. Technol., 2008, 26(5), 562–574 CrossRef CAS PubMed.
- M. T. Zaky and N. H. Mohamed, J. Taiwan Inst. Chem. Eng., 2010, 41, 360–366 CrossRef CAS PubMed.
- F. S. Soliman, M. T. Zaky, A. S. Farag, N. H. Mohamed, L. S. Mohamed and S. Hanafi, Egypt. J. Pet., 2014, 23, 315–321 CrossRef PubMed.
- Annual Book of ASTM-Standards (American Society for Testing and Materials) Petroleum Products, Lubrications, Section 5, West Conshohocken, 1999.
- S. W. Ferris, in Petroleum Waxes, Characterization, Performance and Additives, Technical Association of the Pulp and Paper Industry, Special Technical Association Publication, New York, 1963, pp. 1–19, STAP No. 2 Search PubMed.
- R. I. Gottshall and C. F. McCue, Petroleum Waxes Including Petrolatums, in Criteria for Quality of Petroleum Products, ed. J. P. Allinson, Applied Science Publishers Ltd., on behalf of The Institute of Petroleum, London, 1973, pp. 209–225 Search PubMed.
- L. R. Snyder, in Chromatography, ed. E. Heftmann, Van Nostrand Reinhold Co., New York, 3rd edn, 1975 Search PubMed.
- B. J. Mair and F. D. Rossini, Symposium on Composition of Petroleum Oils, Determination and Evaluation, ASTM STP 224, New Orleans, 1958, p. 9 Search PubMed.
- K. Deutsch, H. J. Kuehn, M. Polzing, I. Deutsch, S. Gruow and J. Stoecker, Prakt. Chem., 1987, 329(4), 681 CrossRef CAS PubMed.
- J. I. Langford and A. J. C. Wilson, J. Appl. Crystallogr., 1978, 11, 102–113 CrossRef CAS.
- A. Sequeria Jr, in Lubricant Base Oil and Wax Processing, Marcel Dekker, Inc., New York, 1994 Search PubMed.
- Koster Keunen Inc., http://www.Kosterkeunen.com.
- US Pharmacopoeia 34 and National Formulary 29, 2011, vol. 3, pp. 3882–3883.
- Ultra Chemical Inc., 1997–2000, http://www.ultrachem.com/productcontent/documents/ultrapure es liquid petrolatum usp-spec.pdf, Copyright.
- G. R. Blackburn, E. N. Ladov, C. R. Mackerer, A. E. Mekitarian and N. Searle, US Patent 5,034,199 A, 1991.
| This journal is © The Royal Society of Chemistry 2015 |