Novel 3-substituted fluorine imidazolium/triazolium salt derivatives: synthesis and antitumor activity†
Jin-Mei Liu‡
a,
Min Wang‡b,
Yun-Jing Zhoua,
Ju-Ming Yanb,
Li-Juan Yang*c,
Yan Li*b,
Hong-Bin Zhanga and
Xiao-Dong Yang*a
aKey Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, School of Chemical Science and Technology, Yunnan University, Kunming, 650091, P. R. China. E-mail: xdyang@ynu.edu.cn; Fax: +86-871-65035538; Tel: +86-871-65031119
bState Key Laboratory for Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Science, Kunming, 650204, P. R. China. E-mail: liyanb@mail.kib.ac.cn
cKey Laboratory of Ethnic Medicine Resource Chemistry, State Ethnic Affairs Commission & Ministry of Education, Yunnan Minzu University, Kunming, 650500, P. R. China. E-mail: yangljyang@sina.com
First published on 21st July 2015
Abstract
A series of novel (±)-3-substituted fluorene–imidazolium/triazolium salt derivatives has been prepared and evaluated in vitro against a panel of human tumor cell lines. The results suggest that the existence of 2-methyl-benzimidazole or 5,6-dimethyl-benzimidazole rings and substitution of the imidazolyl/triazolyl-3/4-position with a naphthylacyl or 4-methoxyphenacyl group were important for modulating cytotoxic activity. Compounds 37 and 42 were found to be the most potent derivatives with IC50 values of 0.51–2.51 μM and exhibited cytotoxic activities selectively against myeloid leukaemia (HL-60), liver carcinoma (SMMC-7721) and lung carcinoma (A549). Compound 37 can remarkably induce the G2/M phase cell cycle arrest and apoptosis in SMMC-7721 cells. Additionally, compound 30 exhibited selective cytotoxicity to some extent between cancer cells (A549) and normal cells (BEAS-2B).
Introduction
Constructing novel pharmacologically interesting hybrid compounds for drug discovery has attracted much attention during the past two decades.1 Fluorenes are an important class of biologically active compounds. Natural products and biologically active agents possessing the fluorene framework display a broad range of biological and pharmacological activities.2 In particular, fluorene derivatives have been identified to possess antitumor activity. As illuminated in Scheme 1, Ixorapeptide I exhibited selective potency against the Hep3B liver cancer cell line,3 while N-(2-(1H-pyrazol-1-yl)phenyl)-7-amino-9-oxo-9H-fluorene-1-carboxamide (PAFC) significantly showed selective cytotoxicity towards breast, colon and hepatocellular carcinoma cells (T47D, HCT116 and SNU398).4On the other hand, imidazolium and triazolium salts have gained considerable interests because of their broad range of biological and pharmacological activity,5 especially antitumor activity.6 For example, two new imidazolium chlorides (Fig. 1), lepidiline A and B, isolated from the roots of Lepidium meyenii, showed potent cytotoxic activity against human cancer cell lines.7 We have previously reported the synthesis of a series of novel imidazolium and triazolium salt derivatives, such as NPTB (Fig. 1), and their potential antitumor activity.8 Studies on molecular mechanisms demonstrated that the imidazolium salt hybrids can induce the G1 phase cell cycle arrest and apoptosis in tumor cells.8c
Considering the potent anticancer activities of fluorene derivatives and imidazolium or triazolium salts, we were interested in synthesizing the hybridizing compounds bearing 3-substituted fluorene and imidazolium or triazolium moieties. To the best of our knowledge, no reports concerning antitumor activity of 3-substituted fluorene–imidazole/triazole hybrid compounds have been found in the literature.
In the present research, a series of novel 3-substituted fluorene–imidazolium/triazolium salt derivatives were synthesized. The purpose of this study was to investigate the antitumor activity of fluorene-based imidazolium/triazolium salt compounds, with the ultimate aim of developing novel potent antitumor agents.
Results and discussion
Chemistry
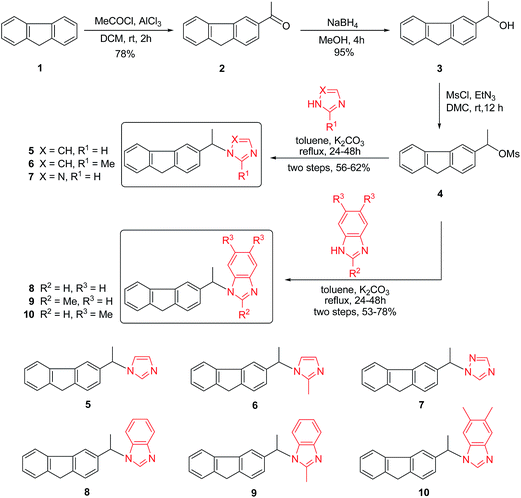
To synthesize 3-substituted fluorene–imidazolium/triazolium salt derivatives, we used commercially available imidazole or triazole derivatives that were alkylated with 1-(9H-fluoren-3-yl)ethanol, which was synthesized from readily available starting materials as shown in Scheme 1. Commercial fluorene 1 was chosen as the starting material for the preparation of a series of 3-substituted fluorene–imidazole/triazole hybrids (5–10). The acetylation of fluorene 1 under Friedel–Craft acylation conditions gave the corresponding 1-(9H-fluoren-3-yl)ethanone 2 in 78% yield. The ketone compounds 2 were reduced via NaBH4 leading to the formation of (±)-1-(9H-fluoren-3-yl)ethanol (3, 95% yield). Subsequently, ethanol 3 was transformed to the respective five (±)-3-substituted fluorene–imidazole hybrids 5, 6, 8–10 with various substituted imidazole or benzimidazole (imidazole, 2-methyl- imidazole, benzimidazole, 2-methyl-benzimidazole or 5,6-dimethyl-benzimidazole) and a (±)-3-substituted fluorene–triazole hybrid 7 with 1,2,4-triazole by refluxing under toluene with 53–78% yields (two steps).Finally, thirty-three (±)-3-substituted fluorene–imidazolium/triazolium salts 11–43 were prepared with excellent yields by reaction of (±)-3-substituted fluorene–imidazole hybrids 5–10 with the corresponding alkyl and phenacyl bromides in refluxing toluene (68–98% yields). The structures and yields of 3-substituted fluorene–imidazole/triazole derivatives are shown in Scheme 2.
 | ||
| Scheme 2 Synthesis of (±)-3-substituted fluorene–imidazolium/triazolium salt derivatives 11–43 from 5–10. | ||
Biological evaluation and structure–activity relationship analysis
The potential cytotoxicity of all newly synthesized (±)-3-substituted fluorene imidazolium/triazolium salt derivatives was evaluated in vitro against a panel of human tumor cell lines according to procedures described in the literature.9 The panel consisted of myeloid leukaemia (HL-60), liver carcinoma (SMMC-7721), lung carcinoma (A549), breast carcinoma (MCF-7) and colon carcinoma (SW480). Cisplatin (DDP) and taxol were used as the reference drugs. The results are summarized in Table 1.| Entry | Compd | Imidazole/triazole ring | R4 | HL-60 | SMMC-7721 | A549 | MCF-7 | SW480 |
|---|---|---|---|---|---|---|---|---|
| a Cytotoxicity as IC50 for each cell line, is the concentration of compound which reduced by 50% the optical density of treated cells with respect to untreated cells using the MTT assay.b Data represent the mean values of three independent determinations. | ||||||||
| 1 | 5 | Imidazole | — | >40 | >40 | >40 | >40 | >40 |
| 2 | 6 | 2-Methyl-imidazole | — | >40 | >40 | >40 | >40 | >40 |
| 3 | 7 | Triazole | — | >40 | >40 | >40 | >40 | >40 |
| 4 | 8 | Benzimidazole | — | >40 | >40 | >40 | >40 | >40 |
| 5 | 9 | 2-Methyl-benzimidazole | — | 10.75 ± 0.85 | 23.36 ± 3.74 | 18.21 ± 0.19 | 36.89 ± 0.91 | 25.92 ± 3.57 |
| 6 | 10 | 5,6-Dimethyl-benzimidazole | — | 31.50 ± 6.37 | >40 | 35.91 ± 1.08 | >40 | >40 |
| 7 | 11 | Imidazole | 4-Bromobenzyl | 2.17 ± 0.22 | 6.76 ± 1.88 | 10.45 ± 0.80 | 4.42 ± 0.15 | 11.94 ± 0.59 |
| 8 | 12 | Imidazole | Phenacyl | 3.49 ± 0.03 | 18.08 ± 0.16 | 23.41 ± 2.25 | 17.68 ± 0.94 | 13.74 ± 0.52 |
| 9 | 13 | Imidazole | 4-Bromophenacyl | 1.75 ± 0.04 | 5.34 ± 0.29 | 4.02 ± 0.35 | 3.03 ± 0.23 | 3.85 ± 0.22 |
| 10 | 14 | Imidazole | 4-Fluorophenacyl | 2.92 ± 0.39 | 16.49 ± 0.44 | 15.29 ± 0.91 | 15.70 ± 1.03 | 12.56 ± 0.89 |
| 11 | 15 | Imidazole | 4-Methoxyphenacyl | 1.10 ± 0.04 | 7.56 ± 0.29 | 9.38 ± 0.82 | 4.52 ± 0.11 | 9.00 ± 0.71 |
| 12 | 16 | Imidazole | Naphthylacyl | 1.01 ± 0.04 | 4.13 ± 0.22 | 3.40 ± 0.49 | 3.17 ± 0.13 | 3.44 ± 0.14 |
| 13 | 17 | 2-Methyl-imidazole | 4-Bromobenzyl | 1.09 ± 0.09 | 4.47 ± 0.46 | 6.75 ± 0.88 | 6.64 ± 1.17 | 11.03 ± 0.48 |
| 14 | 18 | 2-Methyl-imidazole | Phenacyl | 1.47 ± 0.24 | 8.25 ± 0.20 | 11.01 ± 0.33 | 12.35 ± 0.98 | 13.11 ± 0.28 |
| 15 | 19 | 2-Methyl-imidazole | 4-Bromophenacyl | 1.90 ± 0.10 | 8.80 ± 0.35 | 9.06 ± 0.48 | 7.92 ± 0.57 | 12.68 ± 0.92 |
| 16 | 20 | 2-Methyl-imidazole | 4-Methoxyphenacyl | 0.52 ± 0.09 | 2.70 ± 0.81 | 2.86 ± 0.34 | 3.01 ± 0.23 | 10.84 ± 0.44 |
| 17 | 21 | 2-Methyl-imidazole | Naphthylacyl | 0.79 ± 0.01 | 2.65 ± 0.22 | 2.15 ± 0.20 | 2.92 ± 0.06 | 8.89 ± 0.78 |
| 18 | 22 | Triazole | 4-Bromobenzyl | 2.05 ± 0.10 | 8.72 ± 0.07 | 10.00 ± 0.52 | 4.07 ± 0.70 | 11.16 ± 1.19 |
| 19 | 23 | Triazole | Phenacyl | 8.29 ± 1.50 | 17.03 ± 0.65 | 15.34 ± 0.63 | 17.80 ± 0.38 | 16.15 ± 0.30 |
| 20 | 24 | Triazole | 4-Bromophenacyl | 2.07 ± 0.18 | 3.15 ± 0.11 | 2.97 ± 0.16 | 3.41 ± 0.18 | 3.51 ± 0.04 |
| 21 | 25 | Triazole | 4-Methoxyphenacyl | 2.55 ± 0.14 | 13.36 ± 0.50 | 12.32 ± 1.10 | 9.37 ± 2.48 | 11.94 ± 1.94 |
| 22 | 26 | Triazole | Naphthylacyl | 1.70 ± 0.10 | 3.30 ± 0.03 | 3.17 ± 0.04 | 3.41 ± 0.48 | 3.11 ± 0.33 |
| 23 | 27 | Benzimidazole | 4-Bromobenzyl | 0.74 ± 0.05 | 3.42 ± 0.40 | 4.05 ± 0.23 | 2.61 ± 0.08 | 3.14 ± 0.11 |
| 24 | 28 | Benzimidazole | Phenacyl | 0.76 ± 0.05 | 4.54 ± 0.20 | 8.84 ± 1.07 | 3.17 ± 0.15 | 2.89 ± 0.10 |
| 25 | 29 | Benzimidazole | 4-Bromophenacyl | 1.38 ± 0.13 | 3.40 ± 0.13 | 3.01 ± 0.12 | 2.30 ± 0.11 | 3.25 ± 0.10 |
| 26 | 30 | Benzimidazole | 4-Methoxyphenacyl | 0.56 ± 0.02 | 2.22 ± 0.09 | 2.58 ± 0.17 | 1.80 ± 0.18 | 2.54 ± 0.22 |
| 27 | 31 | Benzimidazole | Naphthylacyl | 1.23 ± 0.01 | 3.23 ± 0.11 | 4.04 ± 0.43 | 2.44 ± 0.13 | 3.11 ± 0.14 |
| 28 | 32 | 2-Methyl-benzimidazole | 4-Bromobenzyl | 0.60 ± 0.07 | 2.38 ± 0.14 | 3.64 ± 0.10 | 2.78 ± 0.03 | 2.15 ± 0.13 |
| 29 | 33 | 2-Methyl-benzimidazole | Phenacyl | 0.63 ± 0.15 | 1.97 ± 0.09 | 4.49 ± 0.81 | 2.58 ± 0.01 | 2.43 ± 0.13 |
| 30 | 34 | 2-Methyl-benzimidazole | 4-Bromophenacyl | 0.81 ± 0.04 | 2.43 ± 0.42 | 4.63 ± 0.85 | 3.43 ± 0.02 | 2.82 ± 0.08 |
| 31 | 35 | 2-Methyl-benzimidazole | 4-Fluorophenacyl | 0.68 ± 0.06 | 5.47 ± 0.15 | 9.08 ± 0.65 | 3.12 ± 0.19 | 2.72 ± 0.10 |
| 32 | 36 | 2-Methyl-benzimidazole | 4-Methoxyphenacyl | 0.59 ± 0.03 | 2.04 ± 0.03 | 2.47 ± 0.27 | 2.79 ± 0.09 | 2.28 ± 0.11 |
| 33 | 37 | 2-Methyl-benzimidazole | Naphthylacyl | 0.57 ± 0.02 | 1.38 ± 0.04 | 1.82 ± 0.24 | 2.51 ± 0.13 | 2.36 ± 0.04 |
| 34 | 38 | 5,6-Dimethyl-benzimidazole | 4-Bromobenzyl | 0.45 ± 0.02 | 2.17 ± 0.11 | 2.62 ± 0.06 | 2.99 ± 0.10 | 3.04 ± 0.20 |
| 35 | 39 | 5,6-Dimethyl-benzimidazole | Phenacyl | 0.68 ± 0.07 | 2.44 ± 0.25 | 3.43 ± 0.14 | 3.14 ± 0.08 | 3.28 ± 0.08 |
| 36 | 40 | 5,6-Dimethyl-benzimidazole | 4-Bromophenacyl | 0.58 ± 0.02 | 2.30 ± 0.08 | 3.25 ± 0.45 | 2.79 ± 0.07 | 2.70 ± 0.11 |
| 37 | 41 | 5,6-Dimethyl-benzimidazole | 4-Fluorophenacyl | 1.78 ± 0.13 | 2.82 ± 0.38 | 6.74 ± 0.16 | 3.71 ± 0.31 | 4.43 ± 0.06 |
| 38 | 42 | 5,6-Dimethyl-benzimidazole | 4-Methoxyphenacyl | 0.50 ± 0.03 | 1.69 ± 0.15 | 1.61 ± 0.17 | 2.41 ± 0.18 | 2.41 ± 0.02 |
| 39 | 43 | 5,6-Dimethyl-benzimidazole | Naphthylacyl | 0.87 ± 0.17 | 2.31 ± 0.01 | 2.59 ± 0.13 | 3.02 ± 0.15 | 3.04 ± 0.14 |
| 40 | DDP | 1.16 ± 0.10 | 6.72 ± 0.28 | 7.25 ± 0.46 | 15.06 ± 0.81 | 15.11 ± 0.92 | ||
| 41 | Taxol | <0.008 | <0.008 | <0.008 | <0.008 | <0.008 | ||
As shown in Table 1, the structures of the hybrid compounds have an obvious influence on the inhibitory activities. (±)-3-Substituted fluorene–imidazole/triazole hybrids 5–10 almost lacked activities against all tumor cell lines investigated at the concentration of 40 μM. However, their imidazolium/triazolium salts 11–43 exhibited higher cytotoxic activities. This could be understandable because of the changes of molecular structure, charge distribution and water solubility.10
All imidazolium/triazolium salts 11–43 gave more selectivity towards HL-60, with IC50 values of 0.45–3.49 μM. Among them, nineteen imidazolium salts (19/28) showed higher inhibitory activity against HL-60 cell line than DDP (IC50 values below 1.16 μM). Meanwhile, twenty-four, twenty-two, thirty and thirty-two imidazolium/triazolium salts displayed higher inhibitory activities against SMMC-7721, A549, MCF-7 and SW480 cell lines than DDP. Compounds 38, 37, 42, 30 and 32 showed powerful inhibitory activities selectively against HL-60 (IC50, 0.45 ± 0.02 μM), SMMC-7721 (IC50, 1.38 ± 0.04 μM), A549 (IC50, 1.61 ± 0.17 μM), MCF-7 (IC50, 1.80 ± 0.18 μM) and SW480 (IC50, 2.15 ± 0.13 μM) cell lines, respectively.
In terms of the imidazole ring (imidazole, 2-methyl-imidazole, benzimidazole, 2-methyl-benzimidazole, or 5,6-dimethyl-benzimidazole) and triazole ring, imidazolium salt derivatives 11–16 with imidazole ring, 17–21 with 2-methyl-imidazole and triazolium salts 22–26 exhibited some inhibitory activities. Only compounds 16, 21 and 26, bearing a naphthylacyl substituent at position-3/4 of the imidazole/triazole, showed higher cytotoxic activity compared with DDP with IC50 values of 0.79–8.89 μM. Meanwhile, imidazolium salt derivatives 27–31 with benzimidazole ring displayed medium or high cytotoxic activities. Among them, compounds 30 and 31, bearing a 4-methoxyphenacyl or naphthylacyl substituent at position-3 of the benzimidazole, showed higher cytotoxic activities compared with DDP with IC50 values of 0.56–4.04 μM. However, imidazolium salt derivatives 32–37 with 2-methyl-benzimidazole ring and 38–43 with 5,6-dimethyl-benzimidazole ring displayed powerful cytotoxic activities. All of these kinds of derivatives (12 compounds) were found to be much more active than DDP. Among them, compounds 36, 37, 42 and 43, also bearing a 4-methoxyphenacyl or naphthylacyl substituent at position-3 of the 2-methyl-benzimidazole or 5,6-dimethyl-benzimidazole, exhibited potent cytotoxic activities with IC50 values of 0.50–3.04 μM against five human tumor cell lines investigated.
In terms of the substituent at position-3 of imidazole or position-4 of triazole ring, salts 11, 12, 14, 17, 18, 22, 23, 27, 28, 32, 33, 35, 38, 39 and 41 with a 4-bromobenzyl, phenacyl or 4-fluorophenacyl substituent at position-3/4 of imidazole/triazole ring showed weak activities against five tumor cell lines. Meanwhile, compounds 13, 19, 24, 29, 34 and 40 with a 4-bromophenacyl substituent at position-3/4 of imidazole/triazole ring exhibited medium cytotoxic activities (IC50, 0.58–12.68 μM). However, compared with above benzyl or phenacyl substituent derivatives, imidazolium/triazolium salts with 4-methoxyphenacyl or naphthylacyl group at position-3/4 of imidazole/triazole ring exhibited higher cytotoxic activity. Most of these kinds of derivatives showed moderate or potent activity. Especially, compounds 16, 26, 31, 37 and 43 with a naphthylacyl substituent, as well as compounds 30, 36 and 42 with a 4-methoxyphenacyl substituent at position-3 of the imidazole ring much exhibited higher cytotoxic activity in vitro compared with DDP. Interestingly, compound 37, bearing a naphthylacyl substituent at position-3 of 2-methyl-benzimidazole, and compound 42, bearing a 4-methoxyphenacyl substituent at position-3 of 5,6-dimethyl-benzimidazole, were found to be the most potent derivatives with IC50 values of 0.51–2.51 μM against all of human tumor cell lines investigated and more active than DDP. Notably, compound 37 and 42 displayed cytotoxic activity selectively against HL-60, SMMC-7721 and A549 cell lines with IC50 values below 1.82 μM. This finding shows that steric and electronic effects have an important role in the cytotoxic activity of imidazolium/triazolium salts.
The results suggest that the existence of substituted 2-methyl-benzimidazole and 5,6-dimethyl-benzimidazole ring and substitution of the imidazolyl/triazolyl-3/4-position with a naphthylacyl or 4-methoxyphenacyl group were important for promoting cytotoxic activity. The structure–activity relationship (SAR) results were illustrated in Scheme 3.
 | ||
| Scheme 3 Structure–activity relationship of (±)-3-substituted fluorene–imidazole/triazole derivatives. | ||
Furthermore, we also evaluated the cytotoxicity of the representative compounds 30, 37 and 42 against human normal lung epithelial cell line (BEAS-2B). The results were showed in Table 2. By comparing the IC50 values of the tested compounds towards cancer cell lines with those towards the normal lung epithelial cells BEAS-2B, compound 30 exhibited selective cytotoxicity between cancer and normal cells, with an IC50 value of 16.26 μM against normal BEAS-2B cells, 6.3-fold less toxic than that against lung carcinoma A549 cancer cells. Contrarily, compounds 37 and 42 had not obvious selectivity between cancer and normal cells.
| Entry | Compound no. | BEAS-2B | A549 |
|---|---|---|---|
| 1 | 30 | 16.26 ± 0.33 | 2.58 ± 0.17 |
| 2 | 37 | 3.32 ± 0.05 | 1.82 ± 0.24 |
| 3 | 42 | 2.25 ± 0.04 | 1.61 ± 0.17 |
| 4 | DDP | 9.16 ± 0.23 | 7.25 ± 0.46 |
Compound 37 induces G2/M phase arrest and apoptosis in cancer cells
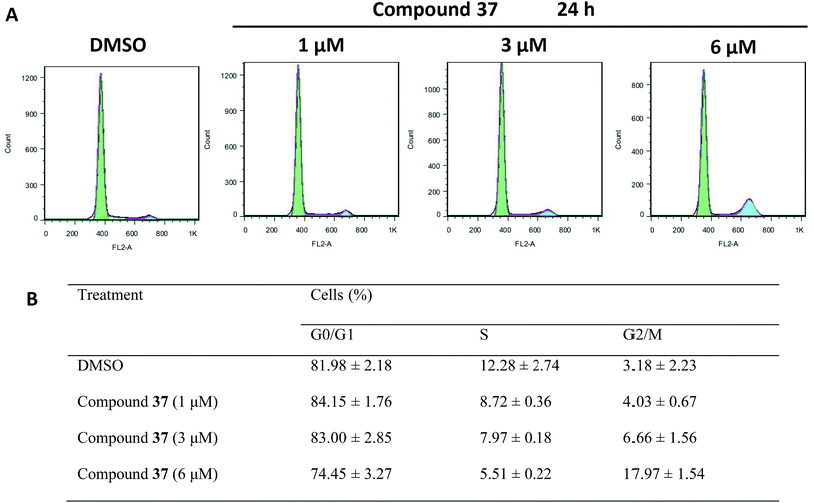
SMMC-7721 cells were exposed to increasing concentrations of compound 37 and cell apoptosis was determined with annexin V-FITC/PI double-labeled cell cytometry. As shown in Fig. 2, after treatment of cells with compound 37 at 1, 3, 6 μM for 48 h, the apoptotic cell rate was 10.04 ± 0.51%, 21.68 ± 0.69% and 56.90 ± 0.99%, respectively, which were statistically different from the control (14.19 ± 0.29%) (Fig. 2). These results showed that 3-substituted fluorene–triazolium salt 37 can remarkably induce apoptosis of the SMMC-7721 cells.The results of cell cycle analysis on SMMC-7721 cells treated with compound 37 were summarized in Fig. 3. Compared with the control cells, the percentage of cells of G2/M phase was increased in the cells incubated with compound 37 with a dose dependent manner. Compound 37 treatment caused 17.97% cells in G2/M phase as compared to control showing 3.18%. In the meanwhile, the fraction of cells in S phase decreased slightly accordingly from 84.15% to 74.45%, while the proportion of G0/G1 phase cells showed no obvious change. The data suggest that compound 37 may induce G2/M phase arrest in the cell cycle. Disruption or malfunction of cell cycle control within the G2/M phase has been recognized as one of the most important biochemical phenomenon for tumor progression and tumorigenesis. The ability of certain small molecules to control cell cycle machinery within the G2/M phase has provided exciting new opportunities with hopes of developing new types of drugs efficacious against refractory cancers.11
Conclusion
In summary, a series of novel (±)-3-substituted fluorene–imidazolium/triazolium salt derivatives prepared proved to be potent antitumor agents. The imidazolium salt derivatives 36, 37, 42 and 43, bearing 2-methyl-benzimidazole or 5,6-dimethyl-benzimidazole ring and a naphthylacyl or 2-naphthylmethyl at position-3 of the imidazole ring, were found to be the most potent compounds. Compound 37, bearing a naphthylacyl substituent at position-3 of 2-methyl-benzimidazole, and compound 42, bearing a 4-methoxyphenacyl substituent at position-3 of 5,6-dimethyl-benzimidazole, were found to be the most potent derivatives with IC50 values of 0.51–2.51 μM against all of human tumor cell lines investigated. Notably, compound 37 and 42 displayed cytotoxic activity selectively against HL-60, SMMC-7721 and A549 cell lines with IC50 values below 1.82 μM. Compound 37 can remarkably induce the G2/M phase cell cycle arrest and apoptosis in SMMC-7721 cells. Interestingly, compound 30 exhibited selective cytotoxicity between cancer and normal cells, which an IC50 value of 16.26 μM against normal BEAS-2B cells, 6.3-fold less toxic than that against lung carcinoma A549 cancer cells. The fluorene-based imidazolium salts 30, 36, 37, 42 and 43 can be considered promising leads for further structural modifications guided by the valuable information derivable from our detailed SARs.Experimental section
General procedures
Melting points were obtained on a XT-4 melting-point apparatus and were uncorrected. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded on a Bruker Avance 300 spectrometer at 300 MHz. Carbon-13 nuclear magnetic resonance (13C-NMR) was recorded on Bruker Avance 300 spectrometer at 75 MHz. Chemical shifts are reported as δ values in parts per million (ppm) relative to tetramethylsilane (TMS) for all recorded NMR spectra. Low-resolution Mass spectra were recorded on a VG Auto Spec-3000 magnetic sector MS spectrometer. High Resolution Mass spectra were taken on AB QSTAR Pulsar mass spectrometer. Elemental analysis were carried out on a Vario-EL analyzer. Silica gel (200–300 mesh) for column chromatography and silica GF254 for TLC were produced by Qingdao Marine Chemical Company (China). All air- or moisture-sensitive reactions were conducted under an argon atmosphere. Starting materials and reagents used in reactions were obtained commercially from Acros, Aldrich, Fluka and were used without purification, unless otherwise indicated.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the product 2 (2.94 g, 78%) as white powder. See ESI† file for characterization data.
1) to afford the product 2 (2.94 g, 78%) as white powder. See ESI† file for characterization data.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the products 3 (2.21 g, 95%) as white powder. See ESI† file for characterization data.
1) to afford the products 3 (2.21 g, 95%) as white powder. See ESI† file for characterization data.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 5–10 in 53–78% yield as white powder. See ESI† file for characterization data.
1) to afford 5–10 in 53–78% yield as white powder. See ESI† file for characterization data.
(±)-1-(1-(9H-Fluoren-3-yl)ethyl)-1H-imidazole (5). Yield 62%. White solid, mp 188–190 °C. IR νmax (cm−1): 2971, 1605, 1498, 1397, 1225, 1085, 740. 1H NMR (300 MHz, CDCl3) δ: 7.70–7.75 (2H, m), 7.62 (1H, s), 7.51 (1H, d, J = 7.2 Hz), 7.31–7.38 (3H, m), 7.16 (1H, d, J = 7.8 Hz), 7.09 (1H, s), 6.95 (1H, s), 5.34–5.41 (1H, m), 3.83 (2H, s), 1.87 (3H, d, J = 6.9 Hz). 13C NMR (75 MHz, CDCl3) δ: 143.97 (C), 143.34 (C), 141.75 (C), 140.94 (C), 140.03 (C), 136.08 (CH), 129.32 (CH), 127.02 (CH), 126.86 (CH), 125.08 (CH), 124.85 (CH), 122.68 (CH), 120.11 (CH), 120.00 (CH), 118.03 (CH), 56.78 (CH), 36.87 (CH2), 22.21 (CH3). Anal. calcd for C18H16N2: C, 83.04; H, 6.19; N, 10.76. Found: C, 82.74; H, 5.81; N, 10.65. HRMS (ESI-TOF) m/z calcd for C18H17N2 [M + H]+ 261.1392, found 261.1395.
(±)-1-(1-(9H-Fluoren-3-yl)ethyl)-2-methyl-1H-imidazole (6). Yield 56%. White solid, mp 134–136 °C. IR νmax (cm−1): 3161, 2974, 1660, 1490, 1268, 1105, 990, 737. 1H NMR (300 MHz, CDCl3) δ: 7.70–7.76 (2H, m), 7.53 (1H, d, J = 7.2 Hz), 7.26–7.39 (2H, m), 7.18 (1H, s), 7.01–7.10 (3H, m), 5.33–5.40 (1H, m), 3.84 (2H, s), 2.31 (3H, s), 1.85 (3H, d, J = 6.9 Hz). 13C NMR (75 MHz, CDCl3) δ: 143.99 (C), 143.31 (C), 141.42 (C), 140.99 (C), 140.31(C), 126.95 (CH), 126.84 (CH), 125.07 (CH), 124.55 (CH), 122.32 (C), 120.09 (CH), 119.95 (CH), 116.78 (CH), 55.27 (CH), 36.89 (CH2), 22.55 (CH3), 13.52 (CH3). Anal. calcd for C19H18N2: C, 83.18; H, 6.61; N, 10.21. Found: C, 83.13; H, 6.65; N, 9.69. HRMS (ESI-TOF) m/z calcd for C19H19N2 [M + H]+ 275.1548, found 275.1553.
(±)-1-(1-(9H-Fluoren-3-yl)ethyl)-1H-1,2,4-triazole (7). Yield 58%. White solid, mp 108–110 °C. IR νmax (cm−1): 3118, 3085, 2968, 2933, 1678, 1614, 1499, 1269, 1140, 1005, 946, 841, 734. 1H NMR (300 MHz, CDCl3) δ: 8.07 (1H, s), 7.99 (1H, s), 7.70–7.74 (2H, m), 7.50 (1H, d, J = 7.2 Hz), 7.40 (1H, s), 7.37 (1H, s), 7.24–7.35 (2H, m), 5.55–5.61 (1H, m), 3.83 (2H, s), 1.95 (3H, d, J = 6.9 Hz). 13C NMR (75 MHz, CDCl3) δ: 151.93 (CH), 144.01 (CH), 143.39 (C), 142.11 (C), 140.91 (C), 138.38 (C), 127.10 (CH), 126.87 (CH), 125.37 (CH), 125.08 (CH), 123.23 (CH), 120.21 (CH), 120.07 (CH), 59.87 (CH), 36.88 (CH2), 21.47 (CH3). Anal. calcd for C17H15N3: C, 78.13; H, 5.79; N, 16.08. Found: C, 78.03; H, 5.72; N, 15.80. HRMS (ESI-TOF) m/z calcd for C17H16N3 [M + H]+ 262.1344, found 262.1349.
(±)-1-(1-(9H-Fluoren-3-yl)ethyl)-1H-benzo[d]imidazole (8). Yield 68%. White solid, mp 179–181 °C. IR νmax (cm−1): 3051, 2970, 1607, 1484, 1223, 744. 1H NMR (300 MHz, CDCl3) δ: 8.15 (1H, s), 7.83 (1H, d, J = 7.8 Hz), 7.71 (2H, dd, J = 15.0, 7.2 Hz), 7.48 (1H, d, J = 6.9 Hz), 7.27–7.36 (3H, m),7.14–7.23 (4H, m), 5.61–5.68 (1H, m), 3.78 (2H, s), 2.00 (3H, d, J = 6.9 Hz). 13C NMR (75 MHz, CDCl3) δ: 144.11 (C), 143.71 (C), 143.35 (C), 141.85 (C), 141.03 (CH), 140.93 (CH), 139.11 (C), 133.64 (C), 127.05 (CH), 126.87 (CH), 125.08 (CH), 124.84 (CH), 122.99 (CH), 122.63 (CH), 122.44 (CH), 120.22 (CH), 120.00 (CH), 110.82 (CH), 55.57 (CH), 36.87 (CH2), 21.80 (CH3). Anal. calcd for C22H18N2: C, 85.13; H, 5.85; N, 9.03. Found: C, 85.03; H, 5.89; N, 8.86. HRMS (ESI-TOF) m/z calcd for C22H19N2 [M + H]+ 311.1548, found 311.1551.
(±)-1-(1-(9H-Fluoren-3-yl)ethyl)-2-methyl-1H-benzo[d]imidazole (9). Yield 53%. White solid, mp 166–168 °C. IR νmax (cm−1): 3048, 2991, 2880, 1609, 1520, 1391, 1283, 1145, 1007, 737. 1H NMR (300 MHz, CDCl3) δ: 7.71–7.76 (3H, m), 7.52 (1H, d, J = 7.2 Hz), 7.25–7.39 (3H, m), 7.16–7.22 (2H, m), 7.07 (2H, d, J = 3.6 Hz), 5.80–5.87 (1H, m), 3.83 (2H, s), 2.64 (3H, s), 2.01 (3H, d, J = 7.2 Hz). 13C NMR (75 MHz, CDCl3) δ: 151.50 (C), 143.97 (C), 143.39 (C), 141.71 (C), 141.57 (C), 140.92 (C), 137.67 (C), 133.67 (C), 127.07 (CH), 126.89 (CH), 125.11 (CH), 124.95 (CH), 123.07 (CH), 122.24 (CH), 122.14 (CH), 120.07 (CH), 118.79 (CH), 111.25 (CH), 53.74 (CH), 36.92 (CH2), 18.84 (CH3), 14.45 (CH3). Anal. calcd for C23H20N2: C, 85.15; H, 6.21; N, 8.63. Found: C, 84.97; H, 6.17; N, 8.36. HRMS (ESI-TOF) m/z calcd for C23H21N2 [M + H]+ 325.1705, found 325.1705.
(±)-1-(1-(9H-Fluoren-3-yl)ethyl)-5,6-dimethyl-1H-benzo[d]imidazole (10). Yield 60%. White solid, mp 182–184 °C. IR νmax (cm−1): 3007, 2969, 2933, 2876, 1617, 1480, 1392, 1224, 1030, 839, 743. 1H NMR (300 MHz, CDCl3) δ: 8.00 (1H, s), 7.73 (2H, t, J = 7.2 Hz), 7.58 (1H, s), 7.50 (1H, d, J = 7.2 Hz), 7.24–7.37 (2H, m), 7.21 (2H, d, J = 7.8 Hz), 6.98 (1H, s), 5.58–5.65 (1H, m), 3.80 (2H, s), 2.33 (3H, s), 2.27 (3H, s), 2.00 (3H, d, J = 7.2 Hz). 13C NMR (75 MHz, CDCl3) δ: 144.04 (C), 143.35 (C), 142.80 (C), 141.66 (C), 141.01 (C), 140.30 (CH), 139.57 (C), 132.31 (C), 131.96 (C), 131.13 (C), 126.97 (CH), 126.84 (CH), 125.06 (CH), 124.77 (CH), 122.55 (CH), 120.33 (CH), 120.16 (CH), 119.96 (CH), 110.77 (CH), 55.25 (CH), 36.87 (CH2), 21.88 (CH3), 20.59 (CH3), 20.22 (CH3). Anal. calcd for C24H22N2: C, 85.17; H, 6.55; N, 8.28. Found: C, 84.79; H, 6.39; N, 7.98. HRMS (ESI-TOF) m/z calcd for C24H23N2 [M + H]+ 339.1861, found 339.1863.
(±)-3-(Naphthalen-2-ylmethyl)-1-(1-(9H-fluoren-3-yl)ethyl)-2-methyl-1H-benzo[d]imidazol-3-ium bromide (37). Yield 80%. White solid, mp 165–167 °C. IR νmax (cm−1): 3021, 1685, 1623, 1520, 1470, 1075, 926, 823, 739. 1H NMR (300 MHz, CDCl3) δ: 9.24 (1H, s), 8.21 (1H, d, J = 8.1 Hz), 8.12 (1H, d, J = 7.2 Hz), 7.92 (1H, d, J = 8.4 Hz), 7.74–7.86 (4H, m), 7.53–7.65 (4H, m), 7.27–7.46 (5H, m), 7.25 (1H, s), 6.95 (2H, s), 6.25–6.31 (1H, m), 3.91 (2H, s), 3.21 (3H, s), 2.21 (3H, d, J = 7.2 Hz). 13C NMR (75 MHz, CDCl3) δ: 190.66 (C), 152.64 (C), 144.58 (C), 143.55 (C), 142.72 (C), 140.49 (C), 136.37 (C), 134.14 (C), 132.60 (C), 132.48 (CH), 130.59 (CH), 130.49 (CH), 129.84 (C), 129.54 (CH), 129.04 (CH), 127.66 (CH), 127.48 (CH), 127.19 (CH), 126.97 (CH), 126.64 (CH), 126.35 (CH), 125.19 (CH), 123.40 (CH), 123.25 (CH), 120.60 (CH), 120.26 (CH), 114.03 (CH), 113.06 (CH), 57.03 (CH), 54.07 (CH2), 36.99 (CH2), 18.60 (CH3), 13.52 (CH3). Anal. calcd for C35H29BrN2O: C, 73.30; H, 5.10; N, 4.88. Found: C, 72.97; H, 5.29; N, 4.47. HRMS (ESI-TOF) m/z calcd for C35H29N2O [M − Br]+ 493.2274, found 493.2277.
(±)-3-(2-(4-Methoxyphenyl)-2-oxoethyl)-1-(1-(9H-fluoren-3-yl)ethyl)-5,6-dimethyl-1H-benzo[d]imidazol-3-ium bromide (42). Yield 96%. White solid, mp 239–241 °C. IR νmax (cm−1): 3191, 2986, 1682, 1597, 1450, 1018, 838, 740. 1H NMR (300 MHz, CDCl3) δ: 11.13 (1H, s), 8.17 (2H, d, J = 8.1 Hz), 7.77 (2H, dd, J = 16.5, 8.4 Hz), 7.61 (1H, s), 7.52 (1H, d, J = 7.2 Hz), 7.30–7.43 (3H, m), 7.28 (1H, s), 7.20 (1H, s), 6.99 (2H, d, J = 8.1 Hz), 6.60 (2H, s), 5.85–5.87 (1H, m), 3.89 (2H, s), 3.88 (3H, s), 2.32 (3H, s), 2.28 (3H, s), 2.25 (3H, d, J = 6.6 Hz). 13C NMR (75 MHz, CDCl3) δ: 188.76 (C), 164.85 (C), 144.69 (C), 143.57 (C), 142.79 (C), 141.24 (CH), 140.60 (C), 137.48 (C), 137.04 (C), 136.20 (C), 131.22 (CH), 129.17 (C), 127.31 (C), 126.86 (CH), 126.62 (CH), 125.14 (CH), 122.91 (CH), 120.61 (CH), 120.14 (CH), 114.44 (CH), 113.56 (CH), 113.11 (CH), 59.25 (CH), 55.66 (CH3), 53.21 (CH2), 36.95 (CH2), 22.30 (CH3), 20.68 (CH3), 20.55 (CH3). Anal. calcd for C33H31BrN2O2: C, 69.84; H, 5.51; N, 4.94. Found: C, 69.77; H, 5.52; N, 4.46. HRMS (ESI-TOF) m/z calcd for C33H31BrN2O2 [M − Br]+ 487.2380, found 487.2389.
Acknowledgements
This work was supported by grants from the Program for Changjiang Scholars and Innovative Research Team in University (IRT13095), Natural Science Foundation of China (21462049, 21332007 and U1402227), Yunnan Province (2013FA028 and 2012FB113) and Education Department of Yunnan Province (ZD2014010), Program for China Scholarship Council (201408535034) and Excellent Young Talents of Yunnan University.Notes and references
- (a) J. J. Walsh and A. Bell, Curr. Pharm. Des., 2009, 15, 2970 CrossRef CAS; (b) C. Viegas Jnr, A. Danuello, V. S. Bolzani, E. J. Barreiro and C. A. M. Fraga, Curr. Med. Chem., 2007, 14, 1829 CrossRef; (c) M. D'hooghe, K. Mollet, R. de Vreese, T. H. M. Jonckers, G. Dams and N. de Kimpe, J. Med. Chem., 2012, 55, 5637 CrossRef PubMed; (d) K. P. Kaliappan and V. Ravikumar, Org. Biomol. Chem., 2005, 3, 848 RSC.
- (a) W. Zhang, Z. Liu, S. Li, Y. Lu, Y. Chen, H. Zhang, G. Zhang, Y. Zhu, G. Zhang, W. Zhang, J. Liu and C. Zhang, J. Nat. Prod., 2012, 75, 1937 CrossRef CAS PubMed; (b) M. Haeubl, S. Schuerz, B. Svejda, L. M. Reith, B. Gruber, R. Pfragner and W. Schoefberger, Eur. J. Med. Chem., 2010, 45, 760 CrossRef CAS PubMed; (c) W. Kemnitzer, N. Sirisoma, B. Nguyen, S. Jiang, S. Kasibhatla, C. Crogan-Grundy, B. Tseng, J. Drewe and S. X. Cai, Bioorg. Med. Chem. Lett., 2009, 19, 3045 CrossRef CAS PubMed; (d) X. Zhang, J. K. Xu, J. Wang, N. L. Wang, H. Kurihara, S. Kitanaka and X. S. Yao, J. Nat. Prod., 2007, 70, 24 CrossRef CAS PubMed.
- C. L. Lee, Y. C. Liao, T. L. Hwang, C. C. Wua, F. R. Chang and Y. C. Wua, Bioorg. Med. Chem. Lett., 2010, 20, 7354 CrossRef CAS PubMed.
- W. Kemnitzer, N. Sirisoma, S. Jiang, S. Kasibhatla, C. Crogan-Grundy, B. Tseng, J. Drewe and S. X. Cai, Bioorg. Med. Chem. Lett., 2010, 20, 1288 CrossRef CAS PubMed.
- (a) F. F. Zhang, C. H. Zhou and L. L. Gan, Bioorg. Med. Chem. Lett., 2010, 20, 1881 CrossRef CAS PubMed; (b) A. Vik, E. Hedner, C. Charnock, L. W. Tangen, Ø. Samuelsen, R. Larsson, L. Bohlinb and L. L. Gundersen, Bioorg. Med. Chem., 2007, 15, 4016 CrossRef CAS PubMed; (c) S. J. Dominianni and T. T. Yen, J. Med. Chem., 1989, 32, 2301 CrossRef CAS; (d) E. E. Alberto, L. L. Rossato, S. H. Alves, D. Alves and A. L. Braga, Org. Biomol. Chem., 2011, 9, 1001 RSC.
- (a) C. G. Fortuna, V. Barresi, G. Berellini and G. Musumarra, Bioorg. Med. Chem., 2008, 16, 4150 CrossRef CAS PubMed; (b) A. Castonguay, C. Doucet, M. Juhas and D. Maysinger, J. Med. Chem., 2012, 55, 8799 CrossRef CAS PubMed.
- B. Cui, B. L. Zheng, K. He and Q. Y. Zheng, J. Nat. Prod., 2003, 66, 1101 CrossRef CAS PubMed.
- (a) C. J. Sun, W. Chen, Y. Li, L. X. Liu, X. Q. Wang, L. J. Li, H. B. Zhang and X. D. Yang, RSC Adv., 2014, 4, 16312 RSC; (b) W. Chen, X. H. Zeng, X. Y. Deng, Y. Li, L. J. Yang, W. C. Wan, X. Q. Wang, H. B. Zhang and X. D. Yang, Bioorg. Med. Chem. Lett., 2013, 23, 4297 CrossRef CAS PubMed; (c) X. H. Zeng, X. D. Yang, Y. L. Zhang, C. Qing and H. B. Zhang, Bioorg. Med. Chem. Lett., 2010, 20, 1844 CrossRef CAS PubMed.
- (a) D.-K. Kim, D. H. Ryu, J. Y. Lee, N. Lee, Y.-W. Kim, J.-S. Kim, K. Chang, G.-J. Im, T.-K. Kim and W.-S. Choi, J. Med. Chem., 2001, 44, 1594 CrossRef CAS PubMed; (b) R. Cao, Q. Chen, X. Hou, H. Chen, H. Guan, Y. Ma, W. Peng and A. Xu, Bioorg. Med. Chem., 2004, 12, 4613 CrossRef CAS PubMed.
- J. Ranke, S. Stolte, R. Störmann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183 CrossRef CAS PubMed.
- (a) Y. Wang, P. Ji, J. Liu, R. R. Broaddus, F. Xue and W. Zhang, Mol. Cancer, 2009, 8, 8 CrossRef PubMed; (b) M. Löbrich and P. A. Jeggo, Nat. Rev. Cancer, 2007, 7, 861 CrossRef PubMed; (c) X. Ling, R. J. Bernacki, M. G. F. Brattain and F. Li, J. Biol. Chem., 2004, 279, 15196 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of experimental procedure, spectral data and copies of the novel compounds. For ESI or other electronic format see DOI: 10.1039/c5ra07947k |
| ‡ These authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2015 |